A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster
- PMID: 11106397
- PMCID: PMC17651
- DOI: 10.1073/pnas.97.25.13772
A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster
Abstract
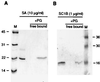
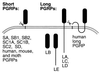
Peptidoglycans from bacterial cell walls trigger immune responses in insects and mammals. A peptidoglycan recognition protein, PGRP, has been cloned from moths as well as vertebrates and has been shown to participate in peptidoglycan-mediated activation of prophenoloxidase in the silk moth. Here we report that Drosophila expresses 12 PGRP genes, distributed in 8 chromosomal loci on the 3 major chromosomes. By analyzing cDNA clones and genomic databases, we grouped them into two classes: PGRP-SA, SB1, SB2, SC1A, SC1B, SC2, and SD, with short transcripts and short 5'-untranslated regions; and PGRP-LA, LB, LC, LD, and LE, with long transcripts and long 5'-untranslated regions. The predicted structures indicate that the first group encodes extracellular proteins and the second group, intracellular and membrane-spanning proteins. Most PGRP genes are expressed in all postembryonic stages. Peptidoglycan injections strongly induce five of the genes. Transcripts from the different PGRP genes were found in immune competent organs such as fat body, gut, and hemocytes. We demonstrate that at least PGRP-SA and SC1B can bind peptidoglycan, and a function in immunity is likely for this family.
Figures





References
-
- Hultmark D. Nature (London) 1994;367:116–117. - PubMed
-
- Brey P T, Hultmark D. Molecular Mechanisms of Immune Responses in Insects. London: Chapman & Hall; 1998.
-
- Engström Y. Dev Comp Immunol. 1999;23:345–358. - PubMed
-
- Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A B. Science. 1999;284:1313–1318. - PubMed
-
- Ghosh S, May M J, Kopp E B. Annu Rev Immunol. 1998;16:225–260. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

