Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice
- PMID: 11106557
- PMCID: PMC1885764
- DOI: 10.1016/S0002-9440(10)64823-7
Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice
Abstract
The potential cytostatic function of apolipoprotein (apo) E in vivo was explored by measuring neointimal hyperplasia in response to vascular injury in apoE-deficient and apoE-overexpressing transgenic mice. Results showed a significant increase in medial thickness, medial area, and neointimal formation after vascular injury in both apoE knockout and wild-type C57BL/6 mice. Immunochemical analysis with smooth muscle alpha-actin-specific antibodies revealed that the neointima contained proliferating smooth muscle cells. Neointimal area was 3.4-fold greater, and the intima/medial ratio as well as stenotic luminal area was more pronounced in apoE(-/-) mice than those observed in control mice (P < 0.05). The human apoE3 transgenic mice in FVB/N genetic background were then used to verify a direct effect of apoE in protection against neointimal hyperplasia in response to mechanically induced vascular injury. Results showed that neointimal area was reduced threefold to fourfold in mice overexpressing the human apoE3 transgene (P < 0.05). Importantly, suppression of neointimal formation in the apoE transgenic mice also abolished the luminal stenosis observed in their nontransgenic FVB/N counterparts. These results documented a direct role of apoE in modulating vascular response to injury, suggesting that increasing apoE level may be beneficial in protection against restenosis after vascular surgery.
Figures


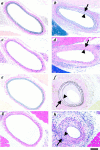



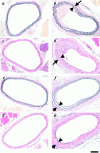
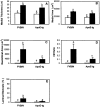
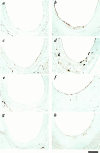
Similar articles
-
Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric oxide synthase.J Lipid Res. 2005 Oct;46(10):2083-90. doi: 10.1194/jlr.M500177-JLR200. Epub 2005 Aug 1. J Lipid Res. 2005. PMID: 16061951 Free PMC article.
-
Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein E-deficient mice.Circulation. 2003 Nov 18;108(20):2491-7. doi: 10.1161/01.CIR.0000099508.76665.9A. Epub 2003 Oct 27. Circulation. 2003. PMID: 14581398
-
Both apolipoprotein E and immune deficiency exacerbate neointimal hyperplasia after vascular injury in mice.Arterioscler Thromb Vasc Biol. 2002 Mar 1;22(3):450-5. doi: 10.1161/hq0302.105377. Arterioscler Thromb Vasc Biol. 2002. PMID: 11884289
-
Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets.Circ Res. 2004 Nov 26;95(11):1125-33. doi: 10.1161/01.RES.0000149518.86865.3e. Epub 2004 Nov 4. Circ Res. 2004. PMID: 15528472
-
Intimal hyperplasia in murine models.Curr Drug Targets. 2008 Mar;9(3):251-60. doi: 10.2174/138945008783755601. Curr Drug Targets. 2008. PMID: 18336244 Free PMC article. Review.
Cited by
-
A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo.J Exp Med. 2003 Jan 6;197(1):41-9. doi: 10.1084/jem.20020945. J Exp Med. 2003. PMID: 12515812 Free PMC article.
-
Hepatic overexpression of the prodomain of furin lessens progression of atherosclerosis and reduces vascular remodeling in response to injury.Atherosclerosis. 2014 Sep;236(1):121-30. doi: 10.1016/j.atherosclerosis.2014.06.015. Epub 2014 Jul 1. Atherosclerosis. 2014. PMID: 25026302 Free PMC article.
-
Lysophosphatidic acid-induced vascular neointimal formation in mouse carotid arteries is mediated by the matricellular protein CCN1/Cyr61.Am J Physiol Cell Physiol. 2016 Dec 1;311(6):C975-C984. doi: 10.1152/ajpcell.00227.2016. Epub 2016 Oct 19. Am J Physiol Cell Physiol. 2016. PMID: 27760754 Free PMC article.
-
Sympathetic nervous system-targeted neuropeptide Y overexpression in mice enhances neointimal formation in response to vascular injury.Peptides. 2009 Apr;30(4):715-20. doi: 10.1016/j.peptides.2008.12.009. Epub 2008 Dec 24. Peptides. 2009. PMID: 19135490 Free PMC article.
-
Progress toward identification of protease activity involved in proteolysis of apolipoprotein e in human brain.J Mol Neurosci. 2004;24(1):73-80. doi: 10.1385/JMN:24:1:073. J Mol Neurosci. 2004. PMID: 15314253 Review.
References
-
- Glagov S: Intimal hyperplasia, vascular modeling, and the restenosis problem. Circulation 1994, 89:2888-2891 - PubMed
-
- Dussaillant GR, Mintz GS, Pichard AD, Kent KM, Satler LF, Popma JJ, Wong SC, Leon MB: Small stent size and intimal hyperplasia contribute to restenosis: a volumetric intravascular ultrasound analysis. J Am Coll Cardiol 1995, 26:720-724 - PubMed
-
- Kearney M, Pieczek A, Haley L, Losordo DW, Andres V, Schainfeld R, Rosenfield K, Isner JM: Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation 1997, 95:1998-2002 - PubMed
-
- Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB: Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 1996, 94:1247-1254 - PubMed
-
- Ono K, Imazu M, Ueda H, Hayashi Y, Shimamoto F, Yamakido M: Differences in histopathologic findings in restenotic lesions after directional coronary atherectomy or balloon angioplasty. Coronary Artery Dis 1998, 9:5-12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

