A feline model of experimentally induced islet amyloidosis
- PMID: 11106586
- PMCID: PMC1885761
- DOI: 10.1016/S0002-9440(10)64852-3
A feline model of experimentally induced islet amyloidosis
Abstract
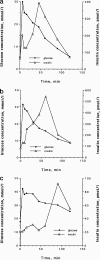
The study of the pathogenesis of islet amyloidosis and its relationship to the development and progression of type 2 diabetes mellitus has been hampered by the lack of an experimentally inducible animal model. The domestic cat, by virtue of the fact that it is one of the few species that spontaneously develop a form of diabetes mellitus that closely resembles human type 2 diabetes, including the formation of amyloid deposits derived from islet amyloid polypeptide (IAPP), was considered to be an excellent candidate species in which to attempt to develop a nontransgenic animal model for this disease process. To develop the model, 8 healthy domestic cats were given a 50% pancreatectomy, which was followed by treatment with growth hormone and dexamethasone. Once a stable diabetic state was established, cats were randomly assigned to groups treated with either glipizide or insulin at doses appropriate to control hyperglycemia. Cats were maintained on this treatment regimen for 18 months and then euthanized. Based on light microscopic examination of Congo red-stained sections of pancreas, all cats were negative for the presence of islet amyloid at the time of pancreatectomy. At the end of the study all 4 glipizide-treated cats had islet amyloid deposits, whereas only 1 of 4 insulin-treated cats had detectable amyloid. In addition, the glipizide treated cats had threefold higher basal and fivefold higher glucose-stimulated plasma IAPP concentrations than insulin-treated cats, suggesting an association between elevated IAPP secretion and islet amyloidosis. Blood-glycosylated hemoglobin concentrations were not significantly different between the two treatment groups. This study documents for the first time an inducible model of islet amyloidosis in a nontransgenic animal.
Figures





References
-
- Westermark P: Quantitative studies of amyloid in the islets of Langerhans. Uppsala J Med Sci 1972, 77:91-94 - PubMed
-
- Schneider HM, Storkel S, Will W: Das Amyloid der Langerhansschen Inseln und seine Beziehung zum Diabetes Mellitus. Dtsch Med Wschr 1980, 105:1143-1147 - PubMed
-
- Johnson KH, O’Brien TD, Westermark P: Islet amyloid, islet amyloid polypeptide and diabetes mellitus. New Engl J Med 1989, 321:513-518 - PubMed
-
- Clark A, de Koning EJ, Hattersley AT, Hansen BC, Yajnik CS, Poulton J: Pancreatic pathology in non-insulin dependent diabetes (NIDDM). Diabetes Res Clin Pract 1995, 28:S39-S47 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

