Nitric oxide and virus infection
- PMID: 11106932
- PMCID: PMC2327086
- DOI: 10.1046/j.1365-2567.2000.00142.x
Nitric oxide and virus infection
Abstract
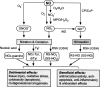
Nitric oxide (NO) has complex and diverse functions in physiological and pathophysiological phenomena. The mechanisms of many events induced by NO are now well defined, so that a fundamental understanding of NO biology is almost established. Accumulated evidence suggests that NO and oxygen radicals such as superoxide are key molecules in the pathogenesis of various infectious diseases. NO biosynthesis, particularly through expression of an inducible NO synthase (iNOS), occurs in a variety of microbial infections. Although antimicrobial activity of NO is appreciated for bacteria and protozoa, NO has opposing effects in virus infections such as influenza virus pneumonia and certain other neurotropic virus infections. iNOS produces an excessive amount of NO for long periods, which allows generation of a highly reactive nitrogen oxide species, peroxynitrite, via a radical coupling reaction of NO with superoxide. Thus, peroxynitrite causes oxidative tissue injury through potent oxidation and nitration reactions of various biomolecules. NO also appears to affect a host's immune response, with immunopathological consequences. For example, overproduction of NO in virus infections in mice is reported to suppress type 1 helper T-cell-dependent immune responses, leading to type 2 helper T-cell-biased immunological host responses. Thus, NO may be a host response modulator rather than a simple antiviral agent. The unique biological properties of NO are further illustrated by our recent data suggesting that viral mutation and evolution may be accelerated by NO-induced oxidative stress. Here, we discuss these multiple roles of NO in pathogenesis of virus infections as related to both non-specific inflammatory responses and immunological host reactions modulated by NO during infections in vivo.
Figures
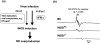
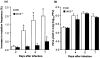

References
-
- Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

