Screening for modulators of spermine tolerance identifies Sky1, the SR protein kinase of Saccharomyces cerevisiae, as a regulator of polyamine transport and ion homeostasis
- PMID: 11113192
- PMCID: PMC88791
- DOI: 10.1128/MCB.21.1.175-184.2001
Screening for modulators of spermine tolerance identifies Sky1, the SR protein kinase of Saccharomyces cerevisiae, as a regulator of polyamine transport and ion homeostasis
Abstract
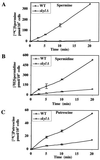
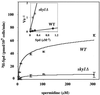
Although most cells are capable of transporting polyamines, the mechanism that regulates polyamine transport in eukaryotes is still largely unknown. Using a genetic screen for clones capable of restoring spermine sensitivity to spermine-tolerant mutants of Saccharomyces cerevisiae, we have demonstrated that Sky1p, a recently identified SR protein kinase, is a key regulator of polyamine transport. Yeast cells deleted for SKY1 developed tolerance to toxic levels of spermine, while overexpression of Sky1p in wild-type cells increased their sensitivity to spermine. Expression of the wild-type Sky1p but not of a catalytically inactive mutant restored sensitivity to spermine. SKY1 disruption results in dramatically reduced uptake of spermine, spermidine, and putrescine. In addition to spermine tolerance, sky1Delta cells exhibit increased tolerance to lithium and sodium ions but somewhat increased sensitivity to osmotic shock. The observed halotolerance suggests potential regulatory interaction between the transport of polyamines and inorganic ions, as suggested in the case of the Ptk2p, a recently described regulator of polyamine transport. We demonstrate that these two kinases act in two different signaling pathways. While deletion or overexpression of SKY1 did not significantly affect Pma1p activity, the ability of overexpressed Sky1p, Ptk1p, and Ptk2p to increase sensitivity to LiCl depends on the integrity of PPZ1 but not of ENA1.
Figures





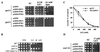
Similar articles
-
Deletions of SKY1 or PTK2 in the Saccharomyces cerevisiae trk1Deltatrk2Delta mutant cells exert dual effect on ion homeostasis.Biochem Biophys Res Commun. 2002 Aug 2;295(5):1142-9. doi: 10.1016/s0006-291x(02)00823-9. Biochem Biophys Res Commun. 2002. PMID: 12135613
-
The STK2 gene, which encodes a putative Ser/Thr protein kinase, is required for high-affinity spermidine transport in Saccharomyces cerevisiae.Mol Cell Biol. 1997 Jun;17(6):2994-3004. doi: 10.1128/MCB.17.6.2994. Mol Cell Biol. 1997. PMID: 9154797 Free PMC article.
-
Role of protein phosphatases 2C on tolerance to lithium toxicity in the yeast Saccharomyces cerevisiae.Mol Microbiol. 2006 Oct;62(1):263-77. doi: 10.1111/j.1365-2958.2006.05370.x. Epub 2006 Aug 31. Mol Microbiol. 2006. PMID: 16956380
-
Potassium and Sodium Transport in Yeast.Adv Exp Med Biol. 2016;892:187-228. doi: 10.1007/978-3-319-25304-6_8. Adv Exp Med Biol. 2016. PMID: 26721275 Review.
-
Ion homeostasis during salt stress in plants.Curr Opin Cell Biol. 2001 Aug;13(4):399-404. doi: 10.1016/s0955-0674(00)00227-1. Curr Opin Cell Biol. 2001. PMID: 11454443 Review.
Cited by
-
Agp2, a member of the yeast amino acid permease family, positively regulates polyamine transport at the transcriptional level.PLoS One. 2013 Jun 3;8(6):e65717. doi: 10.1371/journal.pone.0065717. Print 2013. PLoS One. 2013. PMID: 23755272 Free PMC article.
-
Mechanism of imidazolium ionic liquids toxicity in Saccharomyces cerevisiae and rational engineering of a tolerant, xylose-fermenting strain.Microb Cell Fact. 2016 Jan 20;15:17. doi: 10.1186/s12934-016-0417-7. Microb Cell Fact. 2016. PMID: 26790958 Free PMC article.
-
Predictive evolution of metabolic phenotypes using model-designed environments.Mol Syst Biol. 2022 Oct;18(10):e10980. doi: 10.15252/msb.202210980. Mol Syst Biol. 2022. PMID: 36201279 Free PMC article.
-
The Yeast Permease Agp2 Senses Cycloheximide and Undergoes Degradation That Requires the Small Protein Brp1-Cellular Fate of Agp2 in Response to Cycloheximide.Int J Mol Sci. 2023 Apr 10;24(8):6975. doi: 10.3390/ijms24086975. Int J Mol Sci. 2023. PMID: 37108141 Free PMC article.
-
A genomewide screen for tolerance to cationic drugs reveals genes important for potassium homeostasis in Saccharomyces cerevisiae.Eukaryot Cell. 2011 Sep;10(9):1241-50. doi: 10.1128/EC.05029-11. Epub 2011 Jul 1. Eukaryot Cell. 2011. PMID: 21724935 Free PMC article.
References
-
- Abovich N, Liao X C, Rosbash M. The yeast MUD2 protein: an interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes Dev. 1994;8:843–854. - PubMed
-
- Casero R A, Jr, Mank A R, Saab N H, Wu R, Dyer W J, Woster P M. Growth and biochemical effects of unsymmetrically substituted polyamine analogues in human lung tumor cells 1. Cancer Chemother Pharmacol. 1995;36:69–74. - PubMed
-
- Clotet J, Posas F, de Nadal E, Arino J. The NH2-terminal extension of protein phosphatase PPZ1 has an essential functional role. J Biol Chem. 1996;271:26349–26355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
