Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat
- PMID: 11113306
- PMCID: PMC3129847
- DOI: 10.1016/s0306-4522(00)00386-9
Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat
Abstract
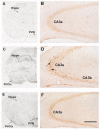
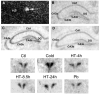
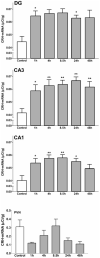
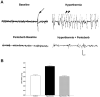
Corticotropin-releasing hormone, a major neuromodulator of the neuroendocrine stress response, is expressed in the immature hippocampus, where it enhances glutamate receptor-mediated excitation of principal cells. Since the peptide influences hippocampal synaptic efficacy, its secretion from peptidergic interneuronal terminals may augment hippocampal-mediated functions such as learning and memory. However, whereas information regarding the regulation of corticotropin-releasing hormone's abundance in CNS regions involved with the neuroendocrine responses to stress has been forthcoming, the mechanisms regulating the peptide's levels in the hippocampus have not yet been determined. Here we tested the hypothesis that, in the immature rat hippocampus, neuronal stimulation, rather than neuroendocrine challenge, influences the peptide's expression. Messenger RNA levels of corticotropin-releasing hormone in hippocampal CA1, CA3 and the dentate gyrus, as well as in the hypothalamic paraventricular nucleus, were determined after cold, a physiological challenge that activates the hypothalamic pituitary adrenal system in immature rats, and after activation of hippocampal neurons by hyperthermia. These studies demonstrated that, while cold challenge enhanced corticotropin-releasing hormone messenger RNA levels in the hypothalamus, hippocampal expression of this neuropeptide was unchanged. Secondly, hyperthermia stimulated expression of hippocampal immediate-early genes, as well as of corticotropin-releasing hormone. Finally, the mechanism of hippocampal corticotropin-releasing hormone induction required neuronal stimulation and was abolished by barbiturate administration. Taken together, these results indicate that neuronal stimulation may regulate hippocampal corticotropin-releasing hormone expression in the immature rat, whereas the peptide's expression in the hypothalamus is influenced by neuroendocrine challenges.
Figures
Similar articles
-
Involvement of serotonergic pathways in mediating the neuronal activity and genetic transcription of neuroendocrine corticotropin-releasing factor in the brain of systemically endotoxin-challenged rats.Neuroscience. 1999 Jan;88(1):223-40. doi: 10.1016/s0306-4522(98)00369-8. Neuroscience. 1999. PMID: 10051203
-
Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus.J Neuroendocrinol. 2003 Nov;15(11):1075-83. doi: 10.1046/j.1365-2826.2003.01100.x. J Neuroendocrinol. 2003. PMID: 14622438
-
Systemic endotoxin increases steady-state gene expression of hypothalamic nitric oxide synthase: comparison with corticotropin-releasing factor and vasopressin gene transcripts.Brain Res. 1995 Dec 24;705(1-2):136-48. doi: 10.1016/0006-8993(95)01142-0. Brain Res. 1995. PMID: 8821744
-
Stress and the developing hippocampus: a double-edged sword?Mol Neurobiol. 2003 Apr;27(2):121-36. doi: 10.1385/MN:27:2:121. Mol Neurobiol. 2003. PMID: 12777683 Free PMC article. Review.
-
Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects.Front Neuroendocrinol. 2006 Jul;27(2):180-92. doi: 10.1016/j.yfrne.2006.02.001. Epub 2006 Apr 17. Front Neuroendocrinol. 2006. PMID: 16603235 Free PMC article. Review.
Cited by
-
Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory.Neurobiol Learn Mem. 2011 Jul;96(1):79-88. doi: 10.1016/j.nlm.2011.02.008. Epub 2011 Feb 19. Neurobiol Learn Mem. 2011. PMID: 21338703 Free PMC article. Review.
-
Neuropeptide Y: potential role in recurrent developmental seizures.Peptides. 2007 Feb;28(2):441-6. doi: 10.1016/j.peptides.2006.08.034. Epub 2006 Dec 29. Peptides. 2007. PMID: 17196709 Free PMC article. Review.
-
Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress.J Neuroendocrinol. 2001 Sep;13(9):799-807. doi: 10.1046/j.1365-2826.2001.00698.x. J Neuroendocrinol. 2001. PMID: 11578530 Free PMC article.
-
How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis.Brain Dev. 2001 Nov;23(7):533-8. doi: 10.1016/s0387-7604(01)00312-6. Brain Dev. 2001. PMID: 11701250 Free PMC article. Review.
-
Microarray analysis of postictal transcriptional regulation of neuropeptides.J Mol Neurosci. 2005;25(3):285-98. doi: 10.1385/JMN:25:3:285. J Mol Neurosci. 2005. PMID: 15800381
References
-
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

