Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis
- PMID: 11114161
- PMCID: PMC14580
- DOI: 10.1073/pnas.98.1.271
Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis
Abstract
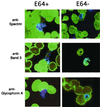
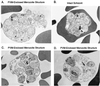
Intraerythrocytic malaria parasites replicate by the process of schizogeny, during which time they copy their genetic material and package it into infective merozoites. These merozoites must then exit the host cell to invade new erythrocytes. To better characterize the events of merozoite escape, erythrocytes containing Plasmodium falciparum schizonts were cultured in the presence of the cysteine protease inhibitor, l-transepoxy-succinyl-leucylamido-(4-guanidino)butane (E64). This treatment resulted in the accumulation of extraerythrocytic merozoites locked within a thin, transparent membrane. Immunomicroscopy demonstrated that the single membrane surrounding the merozoites is not erythrocytic but rather is derived from the parasitophorous vacuolar membrane (PVM). Importantly, structures identical in appearance can be detected in untreated cultures at low frequency. Further studies revealed that (i) merozoites from the PVM-enclosed merozoite structures (PEMS) are invasive, viable, and capable of normal development; (ii) PEMS can be purified easily and efficiently; and (iii) when PEMS are added to uninfected red blood cells, released merozoites can establish a synchronous wave of infection. These observations suggest that l-transepoxy-succinyl-leucylamido-(4-guanidino)butane (E64) causes an accumulation of an intermediate normally present during the process of rupture. We propose a model for the process of rupture: merozoites enclosed within the PVM first exit from the host erythrocyte and then rapidly escape from the PVM by a proteolysis-dependent mechanism.
Figures




Comment in
-
Looking for the exit: How do malaria parasites escape from red blood cells?Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):383-4. doi: 10.1073/pnas.98.2.383. Proc Natl Acad Sci U S A. 2001. PMID: 11209038 Free PMC article. No abstract available.
References
-
- Trigg P I, Kondrachine A V. In: Malaria: Parasite Biology, Pathogenesis and Protection. Sherman I W, editor. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 11–22.
-
- Lyon A J, Haynes J D. J Immunol. 1986;136:2245–2251. - PubMed
-
- Delplace P, Dubremetz J F, Fortier B, Vernes A. Mol Biochem Parasitol. 1985;17:239–251. - PubMed
-
- Bzik D J, Li W B, Horii T, Inselburg J. Mol Biochem Parasitol. 1988;30:279–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

