A recombinant rabies virus expressing vesicular stomatitis virus glycoprotein fails to protect against rabies virus infection
- PMID: 11114165
- PMCID: PMC18978
- DOI: 10.1073/pnas.011510698
A recombinant rabies virus expressing vesicular stomatitis virus glycoprotein fails to protect against rabies virus infection
Abstract
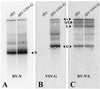
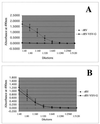
To investigate the importance of the rabies virus (RV) glycoprotein (G) in protection against rabies, we constructed a recombinant RV (rRV) in which the RV G ecto- and transmembrane domains were replaced with the corresponding regions of vesicular stomatitis virus (VSV) glycoprotein (rRV-VSV-G). We were able to recover rRV-VSV-G and found that particle production was equal to rRV. However, the budding of the chimeric virus was delayed and infectious titers were reduced 10-fold compared with the parental rRV strain containing RV G. Biochemical analysis showed equal replication rates of both viruses, and similar amounts of wild-type and chimeric G were present in the respective viral particles. Additional studies were performed to determine whether the immune response against rRV-VSV-G was sufficient to protect against rabies. Mice were primed with rRV or rRV-VSV-G and challenged with a pathogenic strain of RV 12 days later. Similar immune responses against the internal viral proteins of both viruses indicated successful infection. All mice receiving the rRV vaccine survived the challenge, whereas immunization with rRV-VSV-G did not induce protection. The results confirm the crucial role of RV G in an RV vaccine.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

