Autosomal dominant myopathy: missense mutation (Glu-706 --> Lys) in the myosin heavy chain IIa gene
- PMID: 11114175
- PMCID: PMC18967
- DOI: 10.1073/pnas.250289597
Autosomal dominant myopathy: missense mutation (Glu-706 --> Lys) in the myosin heavy chain IIa gene
Abstract
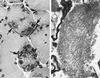
We here report on a human myopathy associated with a mutation in a fast myosin heavy chain (MyHC) gene, and also the genetic defect in a hereditary inclusion body myopathy. The disorder has previously been described in a family with an "autosomal dominant myopathy, with joint contractures, ophthalmoplegia, and rimmed vacuoles." Linkage analysis and radiation hybrid mapping showed that the gene locus (Human Genome Map locus name: IBM3) is situated in a 2-Mb region of chromosome 17p13, where also a cluster of MyHC genes is located. These include the genes encoding embryonic, IIa, IIx/d, IIb, perinatal, and extraocular MyHCs. Morphological analysis of muscle biopsies from patients from the family indicated to us that the type 2A fibers frequently were abnormal, whereas other fiber types appeared normal. This observation prompted us to investigate the MyHC-IIa gene, since MyHC-IIa is the major isoform in type 2A fibers. The complete genomic sequence for this gene was deduced by using an "in silico" strategy. The gene, found to consist of 38 exons, was subjected to a complete mutation scan in patients and controls. We identified a missense mutation, Glu-706 --> Lys, which is located in a highly conserved region of the motor domain, the so-called SH1 helix region. By conformational changes this region communicates activity at the nucleotide-binding site to the neck region, resulting in the lever arm swing. The mutation in this region is likely to result in a dysfunctional myosin, compatible with the disorder in the family.
Figures



References
-
- Darin N, Kyllerman M, Wahlström J, Martinsson T, Oldfors A. Ann Neurol. 1998;44:242–248. - PubMed
-
- Askanas V, Engel W K. Curr Opin Rheumatol. 1998;10:530–542. - PubMed
-
- Weiss A, Schiaffino S, Leinwand L A. J Mol Biol. 1999;290:61–75. - PubMed
-
- Bonne G, Carrier L, Richard P, Hainque B, Schwartz K. Circ Res. 1998;83:580–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

