Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation
- PMID: 11114187
- PMCID: PMC18923
- DOI: 10.1073/pnas.250492197
Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation
Abstract
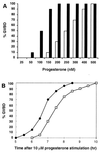
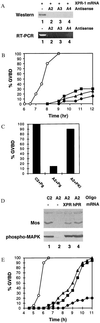
Quiescent full-grown Xenopus oocytes remain arrested at the G(2)/M border of meiosis I until exposed to progesterone, their natural mitogen. Progesterone triggers rapid, nontranscriptional responses that lead to the translational activation of stored mRNAs, resumption of the meiotic cell cycles, and maturation of the oocyte into a fertilizable egg. It has long been presumed that progesterone activates the oocyte through a novel nontranscriptional signaling receptor. Here, we provide evidence that a conventional transcriptional progesterone receptor cloned from Xenopus oocytes, XPR-1, is required for oocyte activation. Overexpression of XPR-1 through mRNA injection increases sensitivity to progesterone and accelerates progesterone-activated cell cycle reentry. Injection of XPR-1 antisense oligonucleotides blocks the ability of oocytes to respond to progesterone; these oocytes are rescued by subsequent injection of XPR-1 or the human progesterone receptor PR-B. Antisense-treated oocytes can be activated in response to inhibition of protein kinase A, one of the earliest known changes occurring downstream of progesterone stimulation. These results argue that the conventional progesterone receptor also functions as the signaling receptor that is responsible for the rapid nontranscriptional activation of frog oocytes.
Figures


Comment in
-
The elusive progesterone receptor in Xenopus oocytes.Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):8-10. doi: 10.1073/pnas.98.1.8. Proc Natl Acad Sci U S A. 2001. PMID: 11136241 Free PMC article. No abstract available.
References
-
- Masui Y, Shibuya E K. In: Molecular Regulation of Nuclear Events in Mitosis and Meiosis. Schlegel R A, Halleck M S, Rao P N, editors. New York: Academic; 1987. pp. 1–42.
-
- Cork R J, Robinson K R. Zygote. 1994;2:289–299. - PubMed
-
- Maller J L. Biol Cell. 1998;90:453–460. - PubMed
-
- Ferrell J E. BioEssays. 1999;21:833–842. - PubMed
-
- Xu L, Glass C K, Rosenfeld M G. Curr Opin Genet Dev. 1999;9:140–147. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

