Regulation of ROMK1 channels by protein-tyrosine kinase and -tyrosine phosphatase
- PMID: 11114300
- PMCID: PMC2822675
- DOI: 10.1074/jbc.M008671200
Regulation of ROMK1 channels by protein-tyrosine kinase and -tyrosine phosphatase
Abstract
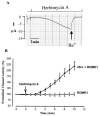
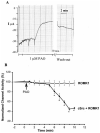
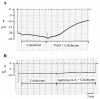
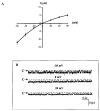
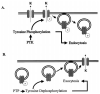
We have used the two-electrode voltage clamp technique and the patch clamp technique to investigate the regulation of ROMK1 channels by protein-tyrosine phosphatase (PTP) and protein-tyrosine kinase (PTK) in oocytes coexpressing ROMK1 and cSrc. Western blot analysis detected the presence of the endogenous PTP-1D isoform in the oocytes. Addition of phenylarsine oxide (PAO), an inhibitor of PTP, reversibly reduced K(+) current by 55% in oocytes coinjected with ROMK1 and cSrc. In contrast, PAO had no significant effect on K(+) current in oocytes injected with ROMK1 alone. Moreover, application of herbimycin A, an inhibitor of PTK, increased K(+) current by 120% and completely abolished the effect of PAO in oocytes coexpressing ROMK1 and cSrc. The effects of herbimycin A and PAO were absent in oocytes expressing the ROMK1 mutant R1Y337A in which the tyrosine residue at position 337 was mutated to alanine. However, addition of exogenous cSrc had no significant effect on the activity of ROMK1 channels in inside-out patches. Moreover, the effect of PAO was completely abolished by treatment of oocytes with 20% sucrose and 250 microg/ml concanavalin A, agents that inhibit the endocytosis of ROMK1 channels. Furthermore, the effect of herbimycin A is absent in the oocytes pretreated with either colchicine, an inhibitor of microtubules, or taxol, an agent that freezes microtubules. We conclude that PTP and PTK play an important role in regulating ROMK1 channels. Inhibiting PTP increases the internalization of ROMK1 channels, whereas blocking PTK stimulates the insertion of ROMK1 channels.
Figures







Similar articles
-
Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct.J Biol Chem. 2000 Jul 7;275(27):20502-7. doi: 10.1074/jbc.M000783200. J Biol Chem. 2000. PMID: 10787405
-
Effects of protein tyrosine kinase and protein tyrosine phosphatase on apical K(+) channels in the TAL.Am J Physiol Cell Physiol. 2001 Oct;281(4):C1188-95. doi: 10.1152/ajpcell.2001.281.4.C1188. Am J Physiol Cell Physiol. 2001. PMID: 11546655
-
Expression of tetraspan protein CD63 activates protein-tyrosine kinase (PTK) and enhances the PTK-induced inhibition of ROMK channels.J Biol Chem. 2008 Mar 21;283(12):7674-81. doi: 10.1074/jbc.M705574200. Epub 2008 Jan 22. J Biol Chem. 2008. PMID: 18211905
-
Regulation of ROMK channels by protein tyrosine kinase and tyrosine phosphatase.Trends Cardiovasc Med. 2002 Apr;12(3):138-42. doi: 10.1016/s1050-1738(02)00155-x. Trends Cardiovasc Med. 2002. PMID: 12007740 Review.
-
The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion.Physiology (Bethesda). 2005 Apr;20:140-6. doi: 10.1152/physiol.00044.2004. Physiology (Bethesda). 2005. PMID: 15772303 Review.
Cited by
-
Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct.Am J Physiol Renal Physiol. 2004 May;286(5):F881-92. doi: 10.1152/ajprenal.00301.2003. Am J Physiol Renal Physiol. 2004. PMID: 15075184 Free PMC article.
-
Regulation of transport in the connecting tubule and cortical collecting duct.Compr Physiol. 2012 Apr;2(2):1541-84. doi: 10.1002/cphy.c110052. Compr Physiol. 2012. PMID: 23227301 Free PMC article. Review.
-
Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter's) knockout mice.J Biol Chem. 2002 Oct 4;277(40):37881-7. doi: 10.1074/jbc.M206644200. Epub 2002 Jul 18. J Biol Chem. 2002. PMID: 12130653 Free PMC article.
-
Src-family protein tyrosine kinase phosphorylates WNK4 and modulates its inhibitory effect on KCNJ1 (ROMK).Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):4495-500. doi: 10.1073/pnas.1503437112. Epub 2015 Mar 24. Proc Natl Acad Sci U S A. 2015. PMID: 25805816 Free PMC article.
-
IKs response to protein kinase A-dependent KCNQ1 phosphorylation requires direct interaction with microtubules.Cardiovasc Res. 2008 Aug 1;79(3):427-35. doi: 10.1093/cvr/cvn085. Epub 2008 Apr 5. Cardiovasc Res. 2008. PMID: 18390900 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

