X-ray spectroscopy-based structure of the Mn cluster and mechanism of photosynthetic oxygen evolution
- PMID: 11115621
- PMCID: PMC3950273
- DOI: 10.1016/s0005-2728(00)00217-6
X-ray spectroscopy-based structure of the Mn cluster and mechanism of photosynthetic oxygen evolution
Abstract
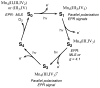
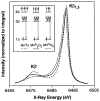
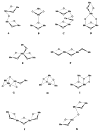
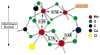
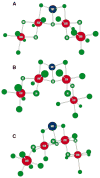
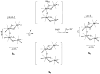
The mechanism by which the Mn-containing oxygen evolving complex (OEC) produces oxygen from water has been of great interest for over 40 years. This review focuses on how X-ray spectroscopy has provided important information about the structure of this Mn complex and its intermediates, or S-states, in the water oxidation cycle. X-ray absorption near-edge structure spectroscopy and high-resolution Mn Kbeta X-ray emission spectroscopy experiments have identified the oxidation states of the Mn in the OEC in each of the intermediate S-states, while extended X-ray absorption fine structure experiments have shown that 2.7 A Mn-Mn di-mu-oxo and 3.3 A Mn-Mn mono-mu-oxo motifs are present in the OEC. X-ray spectroscopy has also been used to probe the two essential cofactors in the OEC, Ca2+ and Cl-, and has shown that Ca2+ is an integral component of the OEC and is proximal to Mn. In addition, dichroism studies on oriented PS II membranes have provided angular information about the Mn-Mn and Mn-Ca vectors. Based on these X-ray spectroscopy data, refined models for the structure of the OEC and a mechanism for oxygen evolution by the OEC are presented.
Figures


Similar articles
-
The Mn cluster in the S(0) state of the oxygen-evolving complex of photosystem II studied by EXAFS spectroscopy: are there three Di-mu-oxo-bridged Mn(2) moieties in the tetranuclear Mn complex?J Am Chem Soc. 2002 Jun 26;124(25):7459-71. doi: 10.1021/ja011621a. J Am Chem Soc. 2002. PMID: 12071755 Free PMC article.
-
Mn K-edge XANES and Kbeta XES studies of two Mn-oxo binuclear complexes: investigation of three different oxidation states relevant to the oxygen-evolving complex of photosystem II.J Am Chem Soc. 2001 Jul 25;123(29):7031-9. doi: 10.1021/ja004306h. J Am Chem Soc. 2001. PMID: 11459481 Free PMC article.
-
Absence of Mn-centered oxidation in the S(2) --> S(3) transition: implications for the mechanism of photosynthetic water oxidation.J Am Chem Soc. 2001 Aug 15;123(32):7804-20. doi: 10.1021/ja004307+. J Am Chem Soc. 2001. PMID: 11493054 Free PMC article.
-
An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and X-ray diffraction signatures.J Biol Inorg Chem. 2002 Jan;7(1-2):2-22. doi: 10.1007/s00775-001-0305-3. Epub 2001 Nov 8. J Biol Inorg Chem. 2002. PMID: 11862536 Review.
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
Cited by
-
QM/MM computational studies of substrate water binding to the oxygen-evolving centre of photosystem II.Philos Trans R Soc Lond B Biol Sci. 2008 Mar 27;363(1494):1149-56; discussion 1156. doi: 10.1098/rstb.2007.2210. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 17971333 Free PMC article. Review.
-
Influence of the 33 kDa manganese-stabilizing protein on the structure and substrate accessibility of the oxygen-evolving complex of photosystem II.Biochemistry. 2005 Jun 21;44(24):8817-25. doi: 10.1021/bi047400+. Biochemistry. 2005. PMID: 15952788 Free PMC article.
-
Development of serial X-ray fluorescence holography for radiation-sensitive protein crystals.J Synchrotron Radiat. 2023 Mar 1;30(Pt 2):368-378. doi: 10.1107/S1600577522011833. Epub 2023 Jan 20. J Synchrotron Radiat. 2023. PMID: 36891850 Free PMC article.
-
Pathway for Mn-cluster oxidation by tyrosine-Z in the S2 state of photosystem II.Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):8723-8. doi: 10.1073/pnas.1401719111. Epub 2014 Jun 2. Proc Natl Acad Sci U S A. 2014. PMID: 24889635 Free PMC article.
-
Current status of the role of Cl(-) ion in the oxygen-evolving complex.Photosynth Res. 2007 Jul-Sep;93(1-3):111-21. doi: 10.1007/s11120-006-9121-5. Epub 2007 Jan 3. Photosynth Res. 2007. PMID: 17200880 Review.
References
-
- Debus RJ. Biochim Biophys Acta. 1992;1102:269–352. - PubMed
-
- Babcock GT, Wikström M. Nature Lond. 1992;356:301–309. - PubMed
-
- Britt RD. In: Oxygenic Photosynthesis: The Light Reactions. Ort DR, Yocum CF, editors. Kluwer Academic; Dordrecht: 1996. pp. 137–164.
-
- Yachandra VK, Sauer K, Klein MP. Chem Rev. 1996;96:2927–2950. - PubMed
-
- Tommos C, Babcock GT. Acc Chem Res. 1998;31:18–25.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

