Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis
- PMID: 11115881
- PMCID: PMC59862
- DOI: 10.1104/pp.124.4.1637
Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis
Abstract
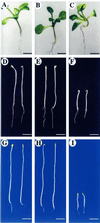
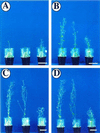
Profilin (PFN) is an ubiquitous, low-M(r), actin-binding protein involved in the organization of the cytoskeleton of eukaryotes including higher plants. PFNs are encoded by a multigene family in Arabidopsis. We have analyzed in vivo functions of Arabidopsis PFN by generating transgenic plants carrying a 35S-PFN-1 or 35S-antisense PFN-1 transgene. Etiolated seedlings underexpressing PFN (PFN-U) displayed an overall dwarf phenotype with short hypocotyls whose lengths were 20% to 25% that of wild type (WT) at low temperatures. Light-grown PFN-U plants were smaller in stature and flowered early. Compared with equivalent cells in WT, most cells in PFN-U hypocotyls and roots were shorter, but more isodiametric, and microscopic observations of etiolated PFN-U hypocotyls revealed a rough epidermal surface. In contrast, light-grown seedlings overexpressing PFN had longer roots and root hair although etiolated seedlings overexpressing PFN were either the same size or slightly longer than WT seedlings. Transgenic seedlings harboring a PFN-1-GUS transgene directed expression in root and root hair and in a ring of cells at the elongating zone of the root tip. As the seedlings matured PFN-1-GUS was mainly expressed in the vascular bundles of cotyledons and leaves. Our results show that Arabidopsis PFNs play a role in cell elongation, cell shape maintenance, polarized growth of root hair, and unexpectedly, in determination of flowering time.
Figures




Similar articles
-
Arabidopsis profilins are functionally similar to yeast profilins: identification of a vascular bundle-specific profilin and a pollen-specific profilin.Plant J. 1996 Aug;10(2):269-79. doi: 10.1046/j.1365-313x.1996.10020269.x. Plant J. 1996. PMID: 8771785
-
Small changes in the regulation of one Arabidopsis profilin isovariant, PRF1, alter seedling development.Plant Cell. 2001 May;13(5):1179-91. doi: 10.1105/tpc.13.5.1179. Plant Cell. 2001. PMID: 11340190 Free PMC article.
-
Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges.Dev Biol. 2000 Nov 15;227(2):618-32. doi: 10.1006/dbio.2000.9908. Dev Biol. 2000. PMID: 11071779
-
The actin cytoskeleton in root hairs: all is fine at the tip.Curr Opin Plant Biol. 2013 Dec;16(6):749-56. doi: 10.1016/j.pbi.2013.10.003. Curr Opin Plant Biol. 2013. PMID: 24446547 Review.
-
Formins: intermediates in signal-transduction cascades that affect cytoskeletal reorganization.Trends Plant Sci. 2002 Nov;7(11):492-8. doi: 10.1016/s1360-1385(02)02341-5. Trends Plant Sci. 2002. PMID: 12417149 Review.
Cited by
-
Profilin is essential for tip growth in the moss Physcomitrella patens.Plant Cell. 2007 Nov;19(11):3705-22. doi: 10.1105/tpc.107.053413. Epub 2007 Nov 2. Plant Cell. 2007. PMID: 17981997 Free PMC article.
-
Arabidopsis CAP regulates the actin cytoskeleton necessary for plant cell elongation and division.Plant Cell. 2002 Jan;14(1):149-63. doi: 10.1105/tpc.010301. Plant Cell. 2002. PMID: 11826305 Free PMC article.
-
A profilin gene promoter from switchgrass (Panicum virgatum L.) directs strong and specific transgene expression to vascular bundles in rice.Plant Cell Rep. 2018 Apr;37(4):587-597. doi: 10.1007/s00299-018-2253-1. Epub 2018 Jan 17. Plant Cell Rep. 2018. PMID: 29340787
-
Global treadmilling coordinates actin turnover and controls the size of actin networks.Nat Rev Mol Cell Biol. 2017 Jun;18(6):389-401. doi: 10.1038/nrm.2016.172. Epub 2017 Mar 1. Nat Rev Mol Cell Biol. 2017. PMID: 28248322 Review.
-
Transcriptional loss of domestication-driven cytoskeletal GhPRF1 gene causes defective floral and fiber development in cotton (Gossypium).Plant Mol Biol. 2021 Dec;107(6):519-532. doi: 10.1007/s11103-021-01200-5. Epub 2021 Oct 4. Plant Mol Biol. 2021. PMID: 34606035
References
-
- Alvarez-Martinez MT, Mani JC, Porte F, Faivre-Sarrailh C, Liautard JP, Sri Widada J. Characterization of the interaction between annexin I and profilin. Eur J Biochem. 1996;238:777–784. - PubMed
-
- Alvarez-Martinez MT, Porte F, Liautard JP, Sri Widada J. Effects of profilin-annexin I association on some properties of both profilin and annexin I: modification of the inhibitory activity of profilin on actin polymerization and inhibition of the self association of annexin I and its interactions with liposomes. Biochim Biophys Acta. 1997;1339:331–340. - PubMed
-
- Baskin TI, Bivens NJ. Stimulation of radial expansion in Arabidopsis roots by inhibitors of actomyosin and vesicle secretion but not by various inhibitors of metabolism. Planta. 1995;197:514–521. - PubMed
-
- Bjorkegren C, Rozycki M, Schutt CE, Lindberg U, Karlsson R. Mutagenesis of human profilin locates its poly (L-proline)-binding site to a hydrophobic patch of aromatic amino acids. FEBS Lett. 1993;333:123–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

