The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast
- PMID: 11115896
- PMCID: PMC59877
- DOI: 10.1104/pp.124.4.1814
The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast
Erratum in
- Plant Physiol 2001 Jul;126(3):1341-2
Abstract
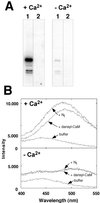
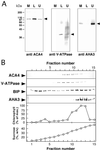
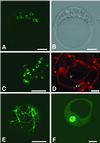
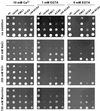
Several lines of evidence suggest that regulation of intracellular Ca(2+) levels is crucial for adaptation of plants to environmental stress. We have cloned and characterized Arabidopsis auto-inhibited Ca(2+)-ATPase, isoform 4 (ACA4), a calmodulin-regulated Ca(2+)-ATPase. Confocal laser scanning data of a green fluorescent protein-tagged version of ACA4 as well as western-blot analysis of microsomal fractions obtained from two-phase partitioning and Suc density gradient centrifugation suggest that ACA4 is localized to small vacuoles. The N terminus of ACA4 contains an auto-inhibitory domain with a binding site for calmodulin as demonstrated through calmodulin-binding studies and complementation experiments using the calcium transport yeast mutant K616. ACA4 and PMC1, the yeast vacuolar Ca(2+)-ATPase, conferred protection against osmotic stress such as high NaCl, KCl, and mannitol when expressed in the K616 strain. An N-terminally modified form of ACA4 specifically conferred increased NaCl tolerance, whereas full-length ATPase had less effect.
Figures



Similar articles
-
ACA12 is a deregulated isoform of plasma membrane Ca²⁺-ATPase of Arabidopsis thaliana.Plant Mol Biol. 2014 Mar;84(4-5):387-97. doi: 10.1007/s11103-013-0138-9. Epub 2013 Oct 8. Plant Mol Biol. 2014. PMID: 24101142 Free PMC article.
-
Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis.FEBS Lett. 2007 Aug 21;581(21):3943-9. doi: 10.1016/j.febslet.2007.07.023. Epub 2007 Jul 23. FEBS Lett. 2007. PMID: 17662727
-
Identification of a calmodulin-regulated soybean Ca(2+)-ATPase (SCA1) that is located in the plasma membrane.Plant Cell. 2000 Aug;12(8):1393-407. doi: 10.1105/tpc.12.8.1393. Plant Cell. 2000. PMID: 10948258 Free PMC article.
-
A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain.J Biol Chem. 1998 Jan 9;273(2):1099-106. doi: 10.1074/jbc.273.2.1099. J Biol Chem. 1998. PMID: 9422775
-
Molecular aspects of higher plant P-type Ca(2+)-ATPases.Biochim Biophys Acta. 2000 May 1;1465(1-2):52-78. doi: 10.1016/s0005-2736(00)00131-0. Biochim Biophys Acta. 2000. PMID: 10748247 Review.
Cited by
-
Calcium efflux systems in stress signaling and adaptation in plants.Front Plant Sci. 2011 Dec 2;2:85. doi: 10.3389/fpls.2011.00085. eCollection 2011. Front Plant Sci. 2011. PMID: 22639615 Free PMC article.
-
Regulation of the major vacuolar Ca²⁺ transporter genes, by intercellular Ca²⁺ concentration and abiotic stresses, in tip-burn resistant Brassica oleracea.Mol Biol Rep. 2013 Jan;40(1):177-88. doi: 10.1007/s11033-012-2047-4. Epub 2012 Nov 9. Mol Biol Rep. 2013. PMID: 23138186
-
Salt tolerance.Arabidopsis Book. 2002;1:e0048. doi: 10.1199/tab.0048. Epub 2002 Sep 30. Arabidopsis Book. 2002. PMID: 22303210 Free PMC article.
-
Regulation of lysosomal ion homeostasis by channels and transporters.Sci China Life Sci. 2016 Aug;59(8):777-91. doi: 10.1007/s11427-016-5090-x. Epub 2016 Jul 19. Sci China Life Sci. 2016. PMID: 27430889 Free PMC article. Review.
-
Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed.Plant Physiol. 2004 Feb;134(2):849-57. doi: 10.1104/pp.103.030023. Epub 2004 Jan 22. Plant Physiol. 2004. PMID: 14739346 Free PMC article.
References
-
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. - PubMed
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. - PubMed
-
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128.
-
- Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPases superfamily. J Mol Evol. 1998;46:84–101. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

