Functional requirement for class I MHC in CNS development and plasticity
- PMID: 11118151
- PMCID: PMC2175035
- DOI: 10.1126/science.290.5499.2155
Functional requirement for class I MHC in CNS development and plasticity
Abstract
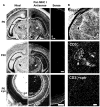
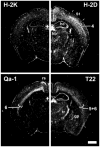
Class I major histocompatibility complex (class I MHC) molecules, known to be important for immune responses to antigen, are expressed also by neurons that undergo activity-dependent, long-term structural and synaptic modifications. Here, we show that in mice genetically deficient for cell surface class I MHC or for a class I MHC receptor component, CD3zeta, refinement of connections between retina and central targets during development is incomplete. In the hippocampus of adult mutants, N-methyl-D-aspartate receptor-dependent long-term potentiation (LTP) is enhanced, and long-term depression (LTD) is absent. Specific class I MHC messenger RNAs are expressed by distinct mosaics of neurons, reflecting a potential for diverse neuronal functions. These results demonstrate an important role for these molecules in the activity-dependent remodeling and plasticity of connections in the developing and mature mammalian central nervous system (CNS).
Figures


Comment in
-
Neuroscience. Immune molecules prune neural links.Science. 2000 Dec 15;290(5499):2051. doi: 10.1126/science.290.5499.2051a. Science. 2000. PMID: 11187818 No abstract available.
-
Is there a flame in the brain in psychosis?Biol Psychiatry. 2014 Feb 15;75(4):258-9. doi: 10.1016/j.biopsych.2013.10.023. Biol Psychiatry. 2014. PMID: 24439553 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

