A new pathway of translational regulation mediated by eukaryotic initiation factor 3
- PMID: 11118224
- PMCID: PMC305884
- DOI: 10.1093/emboj/19.24.6891
A new pathway of translational regulation mediated by eukaryotic initiation factor 3
Abstract
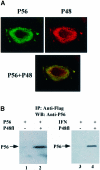
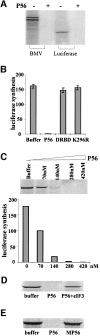
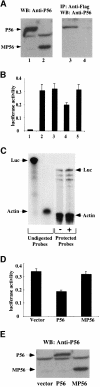
We report a new pathway of translation regulation that may operate in interferon-treated or virus-infected mammalian cells. This pathway is activated by P56, a protein whose synthesis is strongly induced by interferons or double-stranded RNA. Using a yeast two-hybrid screen, we identified the P48 subunit of the mammalian translation initiation factor eIF-3 as a protein that interacts with P56. The P56-P48 interaction was confirmed in human cells by co-immunoprecipitation assays and confocal microscopy. Gel filtration assays revealed that P56 binds to the large eIF-3 complex that contains P48. Purified recombinant P56 inhibited in vitro translation of reporter mRNAs in a dose-dependent fashion, and that inhibition was reversed by the addition of purified eIF-3. In vivo, expression of transfected P56 or induction of the endogenous P56 by interferon caused an inhibition of overall cellular protein synthesis and the synthesis of a transfected reporter protein. As expected, a P56 mutant that does not interact with P48 and eIF-3 failed to inhibit protein synthesis in vitro and in vivo.
Figures
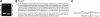

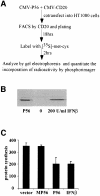
References
-
- Asano K., Merrick,W.C. and Hershey,J.W. (1997) The translation initiation factor eIF3-p48 subunit is encoded by int-6, a site of frequent integration by the mouse mammary tumor virus genome. J. Biol. Chem., 272, 23477–23480. - PubMed
-
- Bandyopadhyay S.K., Leonard,G.T.,Jr, Bandyopadhyay,T., Stark,G.R. and Sen,G.C. (1995) Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J. Biol. Chem., 270, 19624–19629. - PubMed
-
- Bluyssen H.A., Vlietstra,R.J., Faber,P.W., Smit,E.M., Hagemeijer,A. and Trapman,J. (1994) Structure, chromosome localization, and regulation of expression of the interferon-regulated mouse IFi54/IFi56 gene family. Genomics, 24, 137–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

