Actions of hypoxia on catecholamine synthetic enzyme mRNA expression before and after development of adrenal innervation in the sheep fetus
- PMID: 11118487
- PMCID: PMC2270232
- DOI: 10.1111/j.1469-7793.2000.00519.x
Actions of hypoxia on catecholamine synthetic enzyme mRNA expression before and after development of adrenal innervation in the sheep fetus
Abstract
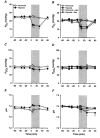
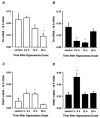
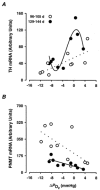
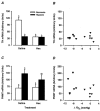
We have investigated adrenal mRNA expression of the catecholamine synthetic enzymes tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT) following acute hypoxia in fetal sheep before (< 105 days gestation, n = 20) and after (> 125 days gestation, n = 20) the development of adrenal innervation and following pretreatment with the nicotinic receptor anatgonist hexamethonium (n = 12). Total RNA was extracted from fetal adrenal glands collected at specific time points at 3-20 h after the onset of either hypoxia ( approximately 50% reduction in fetal arterial oxygen saturation (SO2) for 30 min), or normoxia. Before 105 days, there was a decrease in adrenal TH mRNA expression at 20 h after hypoxia and adrenal TH mRNA expression was directly related to the changes in arterial PO2 measured during normoxia and hypoxia. After 125 days, adrenal TH mRNA levels were suppressed for up to 12 h following hypoxia. In both age groups, adrenal PNMT mRNA expression increased at 3-5 h after hypoxia and was inversely related to the changes in fetal arterial PO2 during normoxia or hypoxia. After 125 days, the administration of hexamethonium (25 mg kg(-1), I.V.) reduced TH mRNA but not PNMT mRNA expression after normoxia. After hexamethonium pretreatment, there was no significant change in either adrenal TH or PNMT mRNA expression following hypoxia. We conclude that acute hypoxia differentially regulates adrenal TH and PNMT mRNA expression in the fetal sheep both before and after the development of adrenal innervation. After the development of adrenal innervation, however, the effect of acute hypoxia upon adrenal TH and PNMT mRNA expression is dependent upon neurogenic input acting via nicotinic receptors.
Figures

References
-
- Adams MB, Phillips ID, Simonetta G, McMillen IC. Differential effects of increasing gestational age and placental restriction on tyrosine hydroxylase, phenylethanolamine N-methyltransferase, and proenkephalin A mRNA levels in the fetal sheep adrenal. Journal of Neurochemistry. 1998;71:394–401. - PubMed
-
- Adams MB, Simonetta G, McMillen IC. The non-neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Brain Research Developmental Brain Research. 1996;94:182–189. - PubMed
-
- Brace RA, Brittingham DS. Fetal vascular pressure and heart rate responses to non-labour uterine contractions. American Journal of Physiology. 1986;251:R409–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

