Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex
- PMID: 11118494
- PMCID: PMC2270226
- DOI: 10.1111/j.1469-7793.2000.00625.x
Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex
Abstract
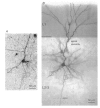
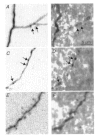
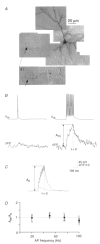
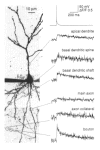
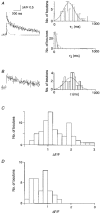
Calcium dynamics associated with a single action potential (AP) were studied in single boutons of the axonal arbor of layer 2/3 pyramidal cells in the neocortex of young (P14-16) rats. We used fluorescence imaging with two-photon excitation and Ca2+-selective fluorescence indicators to measure volume-averaged Ca2+ signals. These rapidly reached a peak (in about 1 ms) and then decayed more slowly (tens to hundreds of milliseconds). Single APs and trains of APs reliably evoked Ca2+ transients in en passant boutons located on axon collaterals in cortical layers 2/3, 4 and 5, indicating that APs propagate actively and reliably throughout the axonal arbor. Branch point failures are unlikely to contribute to differences in synaptic efficacy and reliability in the connections made by layer 2/3 pyramidal cells. AP-evoked Ca2+ transients in boutons were mediated by voltage-dependent Ca2+ channels (VDCCs), predominantly by the P/Q- and N-subtypes. Ca2+ transients were, on average, of significantly larger amplitude in boutons than in the flanking segments of the axon collateral. Large amplitude Ca2+ transients in boutons were spatially restricted to within <= 3 m of axonal length. Single AP-evoked Ca2+ transients varied up to 10-fold across different boutons even if they were located on the same axon collateral. In contrast, variation of Ca2+ transients evoked by successive APs in a given single bouton was small (coefficient of variation, c.v. <= 0.21). Amplitudes of AP-evoked Ca2+ signals did not correlate with the distance of boutons from the soma. In contrast, AP-evoked Ca2+ signals in spines of basal dendrites decreased slightly (correlation coefficient, r2 = -0.27) with distance from the soma. Measurements with the low-affinity Ca2+ indicator Magnesium Green suggest that the volume-averaged residual free [Ca2+]i in a bouton increases on average by 500 nM following a single AP. Higher concentrations of indicator caused, on average, a decrease in the amplitude and an increase in the decay time constant of Ca2+ transients. Assuming a single-compartment model the concentration dependence of decay time constants suggests a low endogenous Ca2+ binding ratio close to 140, indicating that of the total Ca2+ influx ( approximately 2 fC) less than 1% remained free. The indicator concentration dependence of decay time constants further suggests that the residual free Delta[Ca2+]i associated with an AP decays with a time constant of about 60 ms (35 C) reflecting a high Ca2+ extrusion rate of about 2600 s(-1). The results show that AP-evoked volume-averaged Ca2+ transients in single boutons are evoked reliably and, on average, have larger amplitudes than Ca2+ transients in other subcellular compartments of layer 2/3 pyramidal cells. A major functional signature is the large variation in the amplitude of Ca2+ transients between different boutons. This could indicate that local interactions between boutons and different target cells modify the spatiotemporal Ca2+ dynamics in boutons and cause target cell-specific differences in their transmitter release properties.
Figures
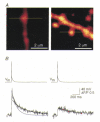



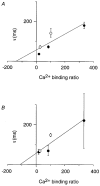
 ). Error bars indicate
). Error bars indicate 
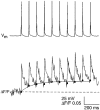

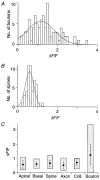
 the range of amplitudes.
the range of amplitudes.

References
-
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
-
- Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. Journal of Neurophysiology. 1995;73:2553–2557. - PubMed
-
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Action-potential propagation by an axonal IA-like K+ conductance in hippocampus. Nature. 1997;389:286–289. - PubMed
-
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

