The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes
- PMID: 11118496
- PMCID: PMC2270229
- DOI: 10.1111/j.1469-7793.2000.00661.x
The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes
Abstract
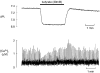
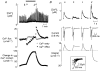
We have investigated the mechanisms responsible for the changes of systolic Ca2+ that occur in voltage-clamped rat ventricular myocytes during acidosis produced by application of the weak acid butyrate (30 mM). Intracellular pH regulation was inhibited with dimethylamiloride (bicarbonate-free solution). The application of butyrate produced an intracellular acidification of 0.33 pH units. This was accompanied by a decrease in systolic Ca2+ to about 50% of control. However, within 2 min, systolic Ca2+ returned to control levels. The decrease in systolic Ca2+ was accompanied by a decrease in the Na+-Ca2+ exchange current observed on repolarisation so that the calculated Ca2+ efflux on Na+-Ca2+ exchange was less than the entry on the L-type Ca2+ current. The magnitude of the Na+-Ca2+ exchange current recovered along with systolic Ca2+ until it equalled the Ca2+ entry on the L-type Ca2+ current. From the measurement of Ca2+ fluxes, it was calculated that, during acidosis, the cell gains 121.6+/-16.2 micromol l(-1) of Ca2+. This is equal to the measured increase of sarcoplasmic reticulum (SR) calcium content obtained by applying caffeine (20 mM) and integrating the resulting Na+-Ca2+ exchange current. We conclude that the recovery of the amplitude of the systolic Ca2+ transient is due to decreased SR calcium release, resulting in reduced Ca2+ efflux from the cell leading to increased SR calcium content.
Figures


References
-
- Buckler KJ, VaughanJones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflügers Archiv. 1990;417:234–239. - PubMed
-
- Choi HS, Trafford AW, Eisner DA. Measurement of calcium entry and exit in quiescent rat ventricular myocytes. Pflügers Archiv. 2000;440:600–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

