Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity
- PMID: 11119506
- PMCID: PMC97872
- DOI: 10.1128/IAI.69.1.194-203.2001
Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity
Abstract
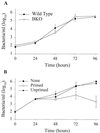
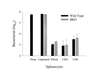
Previous studies have demonstrated a role for B cells, not associated with antibody production, in protection against lethal secondary infection of mice with Francisella tularensis live vaccine strain (LVS). However, the mechanism by which B cells contribute to this protection is not known. To study the specific role of B cells during secondary LVS infection, we developed an in vitro culture system that mimics many of the same characteristics of in vivo infection. Using this culture system, we showed that B cells do not directly control LVS infection but that control of LVS growth is mediated primarily by LVS-primed T cells. Importantly, B cells were not required for the generation of effective memory T cells since LVS-primed, B-cell-deficient (BKO) mice generated CD4(+) and CD8(+) T cells that controlled LVS infection similarly to LVS-primed CD4(+) and CD8(+) T cells from wild-type mice. The control of LVS growth appeared to depend primarily on gamma interferon and nitric oxide and was similar in wild-type and BKO mice. Rather, the inability of BKO mice to survive secondary LVS infection was associated with marked neutrophil influx into the spleen very early after challenge. The neutrophilia was directly associated with B cells, since BKO mice reconstituted with naive B cells prior to a secondary challenge with LVS had decreased bacterial loads and neutrophils in the spleen and survived.
Figures







References
-
- Anthony L S D, Ghadirian E, Nestel F P, Kongshavn P A L. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. - PubMed
-
- Anthony L S D, Kongshavn P A L. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987;2:3–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

