Temperature-regulated protein synthesis by Leptospira interrogans
- PMID: 11119530
- PMCID: PMC97896
- DOI: 10.1128/IAI.69.1.400-404.2001
Temperature-regulated protein synthesis by Leptospira interrogans
Abstract
Leptospira interrogans is an important mammalian pathogen. Transmission from an environmental source requires adaptations to a range of new environmental conditions in the organs and tissues of the infected host. Since many pathogenic bacteria utilize temperature to discern their environment and regulate the synthesis of appropriate proteins, we investigated the effects of temperature on protein synthesis in L. interrogans. Bacteria were grown for several days after culture temperatures were shifted from 30 to 37 degrees C. Triton X-114 cellular fractionation identified several proteins of the cytoplasm, periplasm, and outer membrane for which synthesis was dependent on the culture temperature. Synthesis of a cytoplasmic protein of 20 kDa was switched off at 37 degrees C, whereas synthesis of a 66-kDa periplasmic protein was increased at the higher temperature. Increased synthesis of a 25-kDa outer membrane protein was observed when the organisms were shifted from 30 to 37 degrees C. A 36-kDa protein synthesized at 30 but not at 37 degrees C was identified as LipL36, an outer membrane lipoprotein. In contrast, expression of another lipoprotein, LipL41, was the same at either temperature. Immunoblotting with convalescent equine sera revealed that some proteins exhibiting thermoregulation of synthesis elicited antibody responses during infection. Our results show that sera from horses which aborted as a result of naturally acquired infection with L. interrogans serovar pomona type kennewicki recognize periplasmic and outer membrane proteins which are differentially synthesized in response to temperature and which therefore may be important in the host-pathogen interaction during infection.
Figures
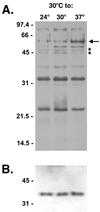

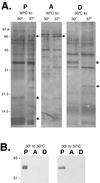


References
-
- Anderson D C, Folland D S, Fox M D, Patton C M, Kaufmann A F. Leptospirosis: a common-source outbreak due to leptospires of the grippotyphosa serogroup. Am J Epidemiol. 1978;107:538–544. - PubMed
-
- Ballard S A, Segers R P, Bleumink-Pluym N, Fyfe J, Faine S, Adler B. Molecular analysis of the hsp (groE) operon of Leptospira interrogans serovar copenhageni. Mol Microbiol. 1993;8:739–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

