Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells
- PMID: 11119537
- PMCID: PMC97903
- DOI: 10.1128/IAI.69.1.456-462.2001
Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells
Abstract
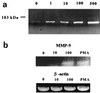
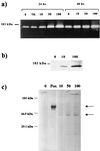
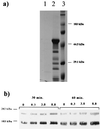
Borrelia burgdorferi, the spirochetal agent of Lyme disease, stimulated human peripheral blood monocytes to release pro-matrix metalloproteinase-9 (gelatinase B; pro-MMP-9) and active matrix metalloproteinase-1 (collagenase-1; MMP-1). Human neutrophils also released pro-MMP-9 and a 130-kDa protein with gelatinolytic activity in response to live B. burgdorferi. In addition, U937 cells and human keratinocyte cells were also stimulated to release pro-MMP-9 under the same conditions. However, human umbilical vein endothelial cells (HUVECs) released pro-MMP-9 and pro-MMP-2 in a constitutive manner and were not influenced by live spirochetes. MMPs produced by human monocytes also enhanced the penetration of B. burgdorferi through extracellular matrix component barriers in vitro. Plasmin stabilized on the surface of the Lyme disease spirochete was shown to activate pro-MMP-9 to its active form. This active form was also observed in the plasma of mice infected with a relapsing fever borrelia. These results suggest that borreliae can upregulate MMPs and possibly mediate an activation cascade initiated by plasmin bound to the microbial surface. MMPs may play a role in dissemination of the Lyme disease spirochete and in the pathogenesis of Borrelia infection.
Figures



References
-
- Anda P, Sanchez-Yebra W, del Mar Vitutia M, Perez Pastrana E, Rodriguez I, Miller N S, Backenson P B, Benach J L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet. 1996;348:162–165. - PubMed
-
- Anthony D C, Ferguson B, Matyzak M K, Miller K M, Esiri M M, Perry V H. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. - PubMed
-
- Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. - PubMed
-
- Blaser J, Triebel S, Maasjosthusmann U, Romisch J, Krahl-Mateblowski U, Freudenberg W, Fricke R, Tschesche H. Determination of metalloproteinases, plasminogen-activators and their inhibitors in the synovial fluids of patients with rheumatoid arthritis during chemical synoviorthesis. Clin Chim Acta. 1996;244:17–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

