Dendritic cells phagocytose and are activated by Treponema pallidum
- PMID: 11119545
- PMCID: PMC97911
- DOI: 10.1128/IAI.69.1.518-528.2001
Dendritic cells phagocytose and are activated by Treponema pallidum
Abstract
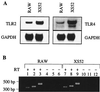
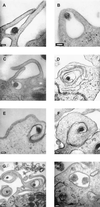
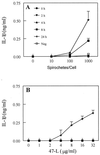
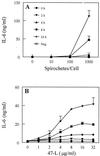
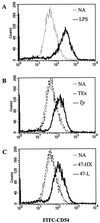
Cell-mediated immune processes play a prominent role in the clinical manifestations of syphilis, a sexually transmitted disease of humans caused by spirochetal bacterium Treponema pallidum. The immune cell type that initiates the early immune response to T. pallidum thus far has not been identified. However, dendritic cells (DCs) are the first immune-competent cells to encounter antigens within skin or mucous membranes, the principal sites of early syphilitic infection. In the present study, immature DC line XS52, derived from murine skin, was utilized to examine T. pallidum-DC interactions and subsequent DC activation (maturation). Electron microscopy revealed that T. pallidum was engulfed by DCs via both coiling and conventional phagocytosis and was delivered to membrane-bound vacuoles. The XS52 DC line expressed surface CD14 and mRNA for Toll-like receptors 2 and 4, molecules comprising important signaling components for immune cell activation by bacterial modulins. Both T. pallidum and a synthetic lipopeptide (corresponding to the 47-kDa major membrane lipoprotein) activated the XS52 DC line, as indicated by the secretion of interleukin-12 (IL-12), IL-1beta, tumor necrosis factor alpha, and IL-6 and elevated surface expression of CD54. The combined data support the contention that DCs stimulated by T. pallidum and/or its proinflammatory membrane lipoproteins are involved in driving the cellular immune processes that typify syphilis.
Figures


References
-
- Aliprantis A O, Yang R-B, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285:736–739. - PubMed
-
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Brightbill H D, Libraty D H, Krutzik S R, Yang R-B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

