Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis
- PMID: 11119601
- PMCID: PMC113925
- DOI: 10.1128/JVI.75.1.311-322.2001
Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis
Abstract
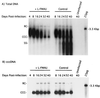
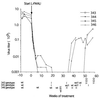
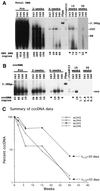
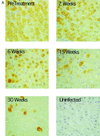
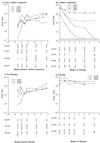
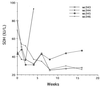
Hepadnaviruses replicate by reverse transcription, which takes place in the cytoplasm of the infected hepatocyte. Viral RNAs, including the pregenome, are transcribed from a covalently closed circular (ccc) viral DNA that is found in the nucleus. Inhibitors of the viral reverse transcriptase can block new DNA synthesis but have no direct effect on the up to 50 or more copies of cccDNA that maintain the infected state. Thus, during antiviral therapy, the rates of loss of cccDNA, infected hepatocytes (1 or more molecules of cccDNA), and replicating DNAs may be quite different. In the present study, we asked how these losses compared when woodchucks chronically infected with woodchuck hepatitis virus were treated with L-FMAU [1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl) uracil], an inhibitor of viral DNA synthesis. Viremia was suppressed for at least 8 months, after which drug-resistant virus began replicating to high titers. In addition, replicating viral DNAs were virtually absent from the liver after 6 weeks of treatment. In contrast, cccDNA declined more slowly, consistent with a half-life of approximately 33 to 50 days. The loss of cccDNA was comparable to that expected from the estimated death rate of hepatocytes in these woodchucks, suggesting that death of infected cells was one of the major routes for elimination of cccDNA. However, the decline in the actual number of infected hepatocytes lagged behind the decline in cccDNA, so that the average cccDNA copy number in infected cells dropped during the early phase of therapy. This observation was consistent with the possibility that some fraction of cccDNA was distributed to daughter cells in those infected hepatocytes that passed through mitosis.
Figures

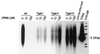
References
-
- Aldrich C E, Coates L, Wu T T, Newbold J, Tennant B C, Summers J, Seeger C, Mason W S. In vitro infection of woodchuck hepatocytes with woodchuck hepatitis virus and ground squirrel hepatitis virus. Virology. 1989;172:247–252. - PubMed
-
- Allen M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrrell D L, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670–1677. - PubMed
-
- Barker L F, Chisari F V, McGrath P P, Dalgard D W, Kirschstein R L, Almeida J D, Edgington T S, Sharp D G, Peterson M R. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973;127:648–652. - PubMed
-
- Bartholomeusz A, Groenen L C, Locarnini S A. Clinical experience with famciclovir against hepatitis B virus and development of resistance. Intervirology. 1997;40:337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

