Functional analysis of the simian immunodeficiency virus Vpx protein: identification of packaging determinants and a novel nuclear targeting domain
- PMID: 11119605
- PMCID: PMC113929
- DOI: 10.1128/JVI.75.1.362-374.2001
Functional analysis of the simian immunodeficiency virus Vpx protein: identification of packaging determinants and a novel nuclear targeting domain
Abstract
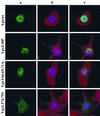
The vpx gene products of human immunodeficiency virus type 2 (HIV-2) and of the closely related simian immunodeficiency viruses from sooty mangabeys (SIVsm) and macaques (SIVmac) comprise a 112-amino-acid virion-associated protein that is critical for efficient virus replication in nondividing cells such as macrophages. When expressed in the absence of other viral proteins, Vpx localizes to the nuclear membrane as well as to the nucleus; however, in the context of virus replication Vpx is packaged into virions via interaction with the p6 domain of the Gag precursor polyprotein (p55(gag)). To identify the domains essential for virion incorporation and nuclear localization, site-directed mutations were introduced into the vpx gene of SIVsmPBj1.9 and functionally analyzed. Our results show that (i) mutation of two highly conserved L74 and I75 residues impaired both virion incorporation and nuclear localization of Vpx; (ii) substitution of conserved H82, G86, C87, P103, and P106 residues impaired Vpx nuclear localization but not virion incorporation; (iii) mutations of conserved Y66, Y69, and Y71 residues impaired virion incorporation but not the translocation of Vpx to the nucleus; and (iv) a mutation at E30 (predicted to disrupt an N-terminal alpha-helix) had no effect on either virion incorporation or nuclear localization of Vpx. Importantly, mutations in Vpx which impaired nuclear localization also reduced virus replication in macaque macrophages, suggesting an important role of the carboxyl terminus of Vpx in nuclear translocation of the viral preintegration complex. Analyzing this domain in greater detail, we identified a 26-amino-acid (aa 60 to 85) fragment that was sufficient to mediate the transport of a heterologous protein (green fluorescent protein [GFP]) to the nucleus. Taken together, these results indicate that virion incorporation and nuclear localization are encoded by two partially overlapping domains in the C-terminus of Vpx (aa 60 to 112). The identification of a novel 26-amino-acid nuclear targeting domain provides a new tool to investigate the nuclear import of the HIV-2/SIV preintegration complex.
Figures


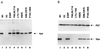





References
-
- Bresnahan P A, Yonemoto W, Greene W C. Cutting edge: SIV Nef protein utilizes both leucine- and tyrosine-based protein sorting pathways for down-regulation of CD4. J Immunol. 1999;163:2977–2981. - PubMed
-
- Chou P Y, Fasman G D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

