Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier
- PMID: 11120756
- PMCID: PMC387254
- DOI: 10.1172/JCI10498
Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier
Abstract
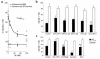
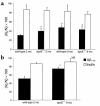
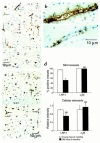
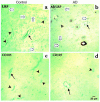
Elimination of amyloid-ss peptide (Ass) from the brain is poorly understood. After intracerebral microinjections in young mice, (125)I-Ass(1-40) was rapidly removed from the brain (t(1/2) </= 25 minutes), mainly by vascular transport across the blood-brain barrier (BBB). The efflux transport system for Ass(1-40) at the BBB was half saturated at 15.3 nM, and the maximal transport capacity was reached between 70 nM and 100 nM. Ass(1-40) clearance was substantially inhibited by the receptor-associated protein, and by antibodies against LDL receptor-related protein-1 (LRP-1) and alpha(2)-macroglobulin (alpha(2)M). As compared to adult wild-type mice, clearance was significantly reduced in young and old apolipoprotein E (apoE) knockout mice, and in old wild-type mice. There was no evidence that Ass was metabolized in brain interstitial fluid and degraded to smaller peptide fragments and amino acids before its transport across the BBB into the circulation. LRP-1, although abundant in brain microvessels in young mice, was downregulated in older animals, and this downregulation correlated with regional Ass accumulation in brains of Alzheimer's disease (AD) patients. We conclude that the BBB removes Ass from the brain largely via age-dependent, LRP-1-mediated transport that is influenced by alpha(2)M and/or apoE, and may be impaired in AD.
Figures



References
-
- Wisniewski T, Ghiso J, Frangione B. Biology of Aβ amyloid in Alzheimer’s disease. Neurobiol Dis. 1997;4:311–328. - PubMed
-
- Selkoe DJ. Alzheimer’s disease: genotype, phenotype, and treatments. Science. 1997;275:630–631. - PubMed
-
- Selkoe DJ. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8:447–453. - PubMed
-
- Younkin SG. The role of Aβ42 in Alzheimer’s disease. J Physiol (Paris) 1998;92:289–292. - PubMed
-
- Roses AD. Alzheimer disease: a model of gene mutations and susceptibility polymorphisms for complex psychiatric diseases. Am J Med Genet. 1998;81:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

