Enhanced peroxynitrite formation is associated with vascular aging
- PMID: 11120770
- PMCID: PMC2213492
- DOI: 10.1084/jem.192.12.1731
Enhanced peroxynitrite formation is associated with vascular aging
Abstract
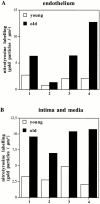
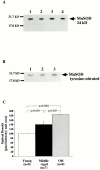
Vascular aging is mainly characterized by endothelial dysfunction. We found decreased free nitric oxide (NO) levels in aged rat aortas, in conjunction with a sevenfold higher expression and activity of endothelial NO synthase (eNOS). This is shown to be a consequence of age-associated enhanced superoxide (.O(2)(-)) production with concomitant quenching of NO by the formation of peroxynitrite leading to nitrotyrosilation of mitochondrial manganese superoxide dismutase (MnSOD), a molecular footprint of increased peroxynitrite levels, which also increased with age. Thus, vascular aging appears to be initiated by augmented.O(2)(-) release, trapping of vasorelaxant NO, and subsequent peroxynitrite formation, followed by the nitration and inhibition of MnSOD. Increased eNOS expression and activity is a compensatory, but eventually futile, mechanism to counter regulate the loss of NO. The ultrastructural distribution of 3-nitrotyrosyl suggests that mitochondrial dysfunction plays a major role in the vascular aging process.
Figures









References
-
- Lüscher T.F., Noll G. The endothelium in coronary vascular control. Heart Dis. 1995;3:1–10.
-
- Palmer R.M., Ashton D.S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. - PubMed
-
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329:2002–2012. - PubMed
-
- Moncada S. The 1991 Ulf von Euler Lecture. The L-argininenitric oxide pathway. Acta Physiol. Scand. 1992;145:201–227. - PubMed
-
- Pollock J.S., Förstermann U., Mitchell J.A., Warner T.D., Schmidt H.H.W., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA. 1991;88:10480–10484. - PMC - PubMed

