Macrolide resistance conferred by base substitutions in 23S rRNA
- PMID: 11120937
- PMCID: PMC90232
- DOI: 10.1128/AAC.45.1.1-12.2001
Macrolide resistance conferred by base substitutions in 23S rRNA
Figures

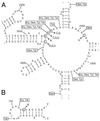
References
-
- Aagaard C, Rosendahl G, Dam M, Powers T, Douthwaite S. Specific structural probing of plasmid-coded ribosomal RNAs from Escherichia coli. Biochimie. 1991;73:1439–1444. - PubMed
-
- Afseth G, Mallavia L P. Copy number of the 16S rRNA gene in Coxiella burnetii. Eur J Epidemiol. 1997;13:729–731. - PubMed
-
- Agrawal R K, Penczek P, Grassucci R A, Li Y, Leith A, Nierhaus K H, Frank J. Direct visualization of A-, P-, and E-site transfer RNAs in the Escherichia coli ribosome. Science. 1996;271:1000–1002. - PubMed
-
- Andersen L P, Kiilerick S, Pedersen G, Thoreson A C, Jorgensen F, Rath J, Larsen N E, Børup O, Krogfelt K, Scheibel J, Rune S. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

