Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy
- PMID: 11120952
- PMCID: PMC90247
- DOI: 10.1128/AAC.45.1.105-116.2001
Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy
Abstract
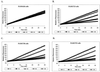
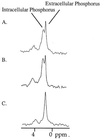
Whole-cell assays were implemented to search for efflux pump inhibitors (EPIs) of the three multidrug resistance efflux pumps (MexAB-OprM, MexCD-OprJ, MexEF-OprN) that contribute to fluoroquinolone resistance in clinical isolates of Pseudomonas aeruginosa. Secondary assays were developed to identify lead compounds with exquisite activities as inhibitors. A broad-spectrum EPI which is active against all three known Mex efflux pumps from P. aeruginosa and their close Escherichia coli efflux pump homolog (AcrAB-TolC) was discovered. When this compound, MC-207,110, was used, the intrinsic resistance of P. aeruginosa to fluoroquinolones was decreased significantly (eightfold for levofloxacin). Acquired resistance due to the overexpression of efflux pumps was also decreased (32- to 64-fold reduction in the MIC of levofloxacin). Similarly, 32- to 64-fold reductions in MICs in the presence of MC-207,110 were observed for strains with overexpressed efflux pumps and various target mutations that confer resistance to levofloxacin (e.g., gyrA and parC). We also compared the frequencies of emergence of levofloxacin-resistant variants in the wild-type strain at four times the MIC of levofloxacin (1 microg/ml) when it was used either alone or in combination with EPI. In the case of levofloxacin alone, the frequency was approximately 10(-7) CFU/ml. In contrast, with an EPI, the frequency was below the level of detection (<10(-11)). In summary, we have demonstrated that inhibition of efflux pumps (i) decreased the level of intrinsic resistance significantly, (ii) reversed acquired resistance, and (iii) resulted in a decreased frequency of emergence of P. aeruginosa strains that are highly resistant to fluoroquinolones.
Figures
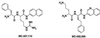
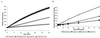



References
-
- Axe D, Bailey J. Application of 31-P nuclear magnetic resonance spectroscopy to investigate plasmid effects on Escherichia coli metabolism. Biotechnol Lett. 1987;9:83–88.
-
- Chen H Y, Yuan M, Livermore D M. Mechanisms of resistance to beta-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J Med Microbiol. 1995;43:300–309. - PubMed
-
- Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A, Bergeron J, Retsema J. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

