Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection
- PMID: 11120955
- PMCID: PMC90250
- DOI: 10.1128/AAC.45.1.129-137.2001
Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection
Abstract
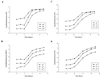
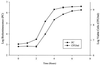
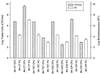
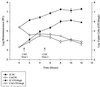
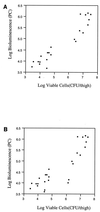
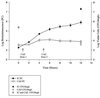
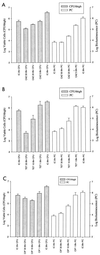
A noninvasive, real-time detection technology was validated for qualitative and quantitative antimicrobial treatment applications. The lux gene cluster of Photorhabdus luminescens was introduced into an Escherichia coli clinical isolate, EC14, on a multicopy plasmid. This bioluminescent reporter bacterium was used to study antimicrobial effects in vitro and in vivo, using the neutropenic-mouse thigh model of infection. Bioluminescence was monitored and measured in vitro and in vivo with an intensified charge-coupled device (ICCD) camera system, and these results were compared to viable-cell determinations made using conventional plate counting methods. Statistical analysis demonstrated that in the presence or absence of antimicrobial agents (ceftazidime, tetracycline, or ciprofloxacin), a strong correlation existed between bioluminescence levels and viable cell counts in vitro and in vivo. Evaluation of antimicrobial agents in vivo could be reliably performed with either method, as each was a sound indicator of therapeutic success. Dose-dependent responses could also be detected in the neutropenic-mouse thigh model by using either bioluminescence or viable-cell counts as a marker. In addition, the ICCD technology was examined for the benefits of repeatedly monitoring the same animal during treatment studies. The ability to repeatedly measure the same animals reduced variability within the treatment experiments and allowed equal or greater confidence in determining treatment efficacy. This technology could reduce the number of animals used during such studies and has applications for the evaluation of test compounds during drug discovery.
Figures

References
-
- Binotto J, MacLachlan R, Sanderson K E. Electrotransformation in Salmonella typhimurium LT2. Can J Microbiol. 1991;37:474–477. - PubMed
-
- Camilli A. Noninvasive techniques for studying pathogenic bacteria in the whole animal. Trends Microbiol. 1996;4:295–296. - PubMed
-
- Casey W M, Nguyen N A. Use of the green fluorescent protein to rapidly assess viability of E. coli in preserved solutions. J Pharm Sci Technol. 1996;50:352–355. - PubMed
-
- Cleeland R, Squires E. Evaluation of new antimicrobials in vitro and in experimental animal infections. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. New York, N.Y: The Williams & Wilkins Co.; 1991. pp. 739–786.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

