Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria
- PMID: 11120963
- PMCID: PMC90258
- DOI: 10.1128/AAC.45.1.181-186.2001
Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria
Abstract
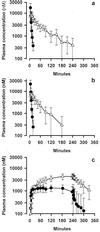
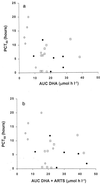
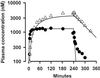
To provide novel data relating to the dispositions, effects, and toxicities of the artemisinin derivatives in severe malaria, we studied 30 Vietnamese adults with slide-positive falciparum malaria treated with intravenous artesunate. Twelve patients with complications (severe; group 1) and 8 patients without complications but requiring parenteral therapy (moderately severe; group 2) received 120 mg of artesunate by injection, and 10 patients with moderately severe complications (group 3) were given 240 mg by infusion. Serial concentrations of artesunate and its active metabolite dihydroartemisinin in plasma were measured by high-performance liquid chromatography. The time to 50% parasite clearance (PCT(50)) was determined from serial parasite densities. Full clinical (including neurological) assessments were performed at least daily. In noncompartmental pharmacokinetic analyses, group mean artesunate half-lives (t(1/2)) were short (range, 2.3 to 4.3 min). The dihydroartemisinin t(1/2) (range, 40 to 64 min), clearance (range, 0.73 to 1.01 liters/h/kg), and volume of distribution (range, 0.77 to 1.01 liters/kg) were also similar both across the three patient groups (P > 0.1) and to previously reported values for patients with uncomplicated malaria. Parasite clearance was prompt (group median PCT(50) range 6 to 9 h) and clinical recovery was complete under all three regimens. These data indicate that the pharmacokinetics of artesunate and dihydroartemisinin are not influenced by the severity of malaria. Since the pharmacokinetic parameters for both artesunate and dihydroartemisinin were similar regardless of whether injection or infusion was used, artesunate can be considered a prodrug that is converted stoichiometrically to dhydroartemisinin. Conventional doses of artesunate are safe and effective when given to patients with complications of falciparum malaria.
Figures
References
-
- Barradell L B, Fitton A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. - PubMed
-
- Batty K T. Pharmacokinetic studies of artesunate and dihydroartemisinin, p. 114–116. Ph.D. thesis. Nedlands, Australia: University of Western Australia; 1999.
-
- Batty K T, Davis T M E, Thu L T A, Binh T Q, Anh T K, Ilett K F. Selective high-performance liquid chromatographic determination of artesunate and α- and β-dihydroartemisinin in patients with falciparum malaria. J Chromatogr B. 1996;677:345–350. - PubMed
-
- Batty K T, Ilett K F, Davis T M E. Chemical stability of artesunate injection and proposal for its administration by intravenous infusion. J Pharm Pharmacol. 1996;48:22–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

