Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1)
- PMID: 11121022
- PMCID: PMC18885
- DOI: 10.1073/pnas.97.26.14145
Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1)
Abstract
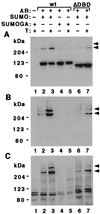
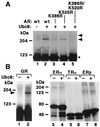
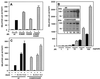
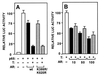
Modification by SUMO-1 is proposed to play a role in protein targeting and/or stability. The SUMO-1-conjugating enzyme Ubc9 interacts with androgen receptor (AR), a ligand-activated transcription factor belonging to the steroid receptor superfamily. We show here that AR is covalently modified by SUMO-1 (sumoylated) in an androgen-enhanced fashion and identify the principal acceptor site in the N-terminal domain of AR. Substitutions of sumoylated Lys residues enhanced transcriptional activity of AR without influencing its transrepressing activity. Interestingly, the same Lys residues form the cores of the recently described transcriptional synergy control motifs in AR [Iñiguez-Lluhi, J. A. & Pearce, D. (2000) Mol. Cell. Biol. 20, 6040-6050]. These motifs, which match perfectly with the sumoylation consensus sequence, are also present in the N-terminal domains of glucocorticoid, mineralocorticoid, and progesterone receptor. Taken together, our data suggest that reversible sumoylation is a mechanism for regulation of steroid receptor function.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

