c-Mos forces the mitotic cell cycle to undergo meiosis II to produce haploid gametes
- PMID: 11121036
- PMCID: PMC18913
- DOI: 10.1073/pnas.97.26.14301
c-Mos forces the mitotic cell cycle to undergo meiosis II to produce haploid gametes
Abstract
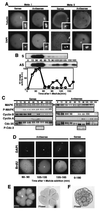
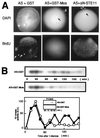
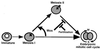
The meiotic cycle reduces ploidy through two consecutive M phases, meiosis I and meiosis II, without an intervening S phase. To maintain ploidy through successive generations, meiosis must be followed by mitosis after the recovery of diploidy by fertilization. However, the coordination from meiotic to mitotic cycle is still unclear. Mos, the c-mos protooncogene product, is a key regulator of meiosis in vertebrates. In contrast to the previous observation that Mos functions only in vertebrate oocytes that arrest at meiotic metaphase II, here we isolate the first invertebrate mos from starfish and show that Mos functions also in starfish oocytes that arrest after the completion of meiosis II but not at metaphase II. In the absence of Mos, meiosis I is followed directly by repeated embryonic mitotic cycles, and its reinstatement restores meiosis II and subsequent cell cycle arrest. These observations imply that after meiosis I, oocytes have a competence to progress through the embryonic mitotic cycle, but that Mos diverts the cell cycle to execute meiosis II and remains to restrain the return to the mitotic cycle. We propose that a role of Mos that is conserved in invertebrate and vertebrate oocytes is not to support metaphase II arrest but to prevent the meiotic/mitotic conversion after meiosis I until fertilization, directing meiosis II to ensure the reduction of ploidy.
Figures


Similar articles
-
Mos is not required for the initiation of meiotic maturation in Xenopus oocytes.EMBO J. 2002 Aug 1;21(15):4026-36. doi: 10.1093/emboj/cdf400. EMBO J. 2002. PMID: 12145203 Free PMC article.
-
Meiotic cell cycle in Xenopus oocytes is independent of cdk2 kinase.EMBO J. 1997 Jul 1;16(13):3860-5. doi: 10.1093/emboj/16.13.3860. EMBO J. 1997. PMID: 9233796 Free PMC article.
-
Disrupted MOS signaling alters meiotic cell cycle regulation and the egg transcriptome.Reproduction. 2025 Jun 9;170(1):e250156. doi: 10.1530/REP-25-0156. Print 2025 Jul 1. Reproduction. 2025. PMID: 40424049 Free PMC article.
-
Synthesis and function of Mos: the control switch of vertebrate oocyte meiosis.Bioessays. 1997 Jan;19(1):23-8. doi: 10.1002/bies.950190106. Bioessays. 1997. PMID: 9008414 Review.
-
Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs.Genes Dev. 2003 Mar 15;17(6):683-710. doi: 10.1101/gad.1071303. Genes Dev. 2003. PMID: 12651887 Review. No abstract available.
Cited by
-
Initiation of DNA replication after fertilization is regulated by p90Rsk at pre-RC/pre-IC transition in starfish eggs.Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):5006-11. doi: 10.1073/pnas.1000587107. Epub 2010 Feb 25. Proc Natl Acad Sci U S A. 2010. PMID: 20185755 Free PMC article.
-
Managing the Oocyte Meiotic Arrest-Lessons from Frogs and Jellyfish.Cells. 2020 May 7;9(5):1150. doi: 10.3390/cells9051150. Cells. 2020. PMID: 32392797 Free PMC article. Review.
-
MPF-based meiotic cell cycle control: Half a century of lessons from starfish oocytes.Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(4):180-203. doi: 10.2183/pjab.94.013. Proc Jpn Acad Ser B Phys Biol Sci. 2018. PMID: 29643273 Free PMC article. Review.
-
The first embryo, the origin of cancer and animal phylogeny. I. A presentation of the neoplastic process and its connection with cell fusion and germline formation.Front Cell Dev Biol. 2023 Jan 4;10:1067248. doi: 10.3389/fcell.2022.1067248. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684435 Free PMC article.
-
Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles.EMBO J. 2003 Oct 15;22(20):5633-42. doi: 10.1093/emboj/cdg535. EMBO J. 2003. PMID: 14532135 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

