Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response
- PMID: 11121051
- PMCID: PMC18946
- DOI: 10.1073/pnas.97.26.14488
Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response
Abstract
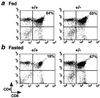
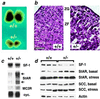
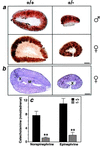
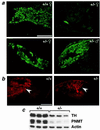
Adrenal steroids are essential for homeostasis and survival during severe physiological stress. Analysis of a patient heterozygous for the steroidogenic factor-1 (SF-1) gene suggested that reduced expression of this nuclear receptor leads to adrenal failure. We therefore examined SF-1 heterozygous (+/-) mice as a potential model for delineating mechanisms underlying this disease. Here we show that SF-1 +/- mice exhibit adrenal insufficiency resulting from profound defects in adrenal development and organization. However, compensatory mechanisms, such as cellular hypertrophy and increased expression of the rate-limiting steroidogenic protein StAR, help to maintain adrenal function at near normal capacity under basal conditions. In contrast, adrenal deficits in SF-1 heterozygotes are revealed under stressful conditions, demonstrating that normal gene dosage of SF-1 is required for mounting an adequate stress response. Our findings predict that natural variations leading to reduced SF-1 function may underlie some forms of subclinical adrenal insufficiency, which become life threatening during traumatic stress.
Figures

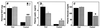
References
-
- Oelkers W. N Engl J Med. 1996;335:1206–1212. - PubMed
-
- Orth D, Kovacs W. In: Williams Textbook of Endocrinology. Wilson J D, Foster D W, editors. Philadelphia: Saunders; 1992. pp. 853–951.
-
- Zanaria E, Muscatelli F, Bardoni B, Strom T M, Guioli S, Guo W, Lalli E, Moser C, Walker A P, McCabe E R, et al. Nature (London) 1994;372:635–641. - PubMed
-
- Merke D P, Tajima T, Baron J, Cutler G B., Jr N Engl J Med. 1999;340:1248–1252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

