Telokin expression is restricted to smooth muscle tissues during mouse development
- PMID: 11121372
- PMCID: PMC2860107
- DOI: 10.1152/ajpcell.2001.280.1.C12
Telokin expression is restricted to smooth muscle tissues during mouse development
Abstract
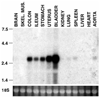
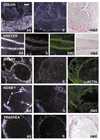
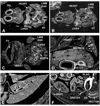
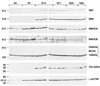
Telokin is a 17-kDa protein with an amino acid sequence that is identical to the COOH terminus of the 130-kDa myosin light chain kinase (MLCK). Telokin mRNA is transcribed from a second promoter, located within an intron, in the 3' region of the MLCK gene. In the current study, we show by in situ mRNA hybridization that telokin mRNA is restricted to the smooth muscle cell layers within adult smooth muscle tissues. In situ mRNA analysis of mouse embryos also revealed that telokin expression is restricted to smooth muscle tissues during embryonic development. Telokin mRNA expression was first detected in mouse gut at embryonic day 11.5; no telokin expression was detected in embryonic cardiac or skeletal muscle. Expression of telokin was also found to be regulated during postnatal development of the male and female reproductive tracts. In both uterus and vas deferens, telokin protein expression greatly increased between days 7 and 14 of postnatal development. The increase in telokin expression correlated with an increase in the expression of several other smooth muscle-restricted proteins, including smooth muscle myosin and alpha-actin.
Figures


Similar articles
-
The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin.J Biol Chem. 1991 Dec 15;266(35):23945-52. J Biol Chem. 1991. PMID: 1748667 Free PMC article.
-
Telokin expression is mediated by a smooth muscle cell-specific promoter.Am J Physiol. 1996 Jun;270(6 Pt 1):C1656-65. doi: 10.1152/ajpcell.1996.270.6.C1656. Am J Physiol. 1996. PMID: 8764148
-
Activation of the smooth muscle-specific telokin gene by thyrotroph embryonic factor (TEF).J Biol Chem. 2004 Apr 16;279(16):15929-37. doi: 10.1074/jbc.M313822200. Epub 2004 Jan 26. J Biol Chem. 2004. PMID: 14702338
-
Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues.Am J Physiol Cell Physiol. 2006 Nov;291(5):C817-27. doi: 10.1152/ajpcell.00198.2006. Epub 2006 Jun 14. Am J Physiol Cell Physiol. 2006. PMID: 16774989 Free PMC article. Review.
-
Regulation of the cross-bridge cycle: the effects of MgADP, LC17 isoforms and telokin.Acta Physiol Scand. 1998 Dec;164(4):381-8. doi: 10.1111/j.1365-201x.1998.tb10695.x. Acta Physiol Scand. 1998. PMID: 9887962 Review.
Cited by
-
microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart.Elife. 2013 Nov 19;2:e01323. doi: 10.7554/eLife.01323. Elife. 2013. PMID: 24252873 Free PMC article.
-
Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1.Mol Cell Biol. 2005 Nov;25(22):9874-85. doi: 10.1128/MCB.25.22.9874-9885.2005. Mol Cell Biol. 2005. PMID: 16260603 Free PMC article.
-
Vascular smooth muscle phenotypic diversity and function.Physiol Genomics. 2010 Nov 15;42A(3):169-87. doi: 10.1152/physiolgenomics.00111.2010. Epub 2010 Aug 24. Physiol Genomics. 2010. PMID: 20736412 Free PMC article. Review.
-
Regulation of 130-kDa smooth muscle myosin light chain kinase expression by an intronic CArG element.J Biol Chem. 2013 Nov 29;288(48):34647-57. doi: 10.1074/jbc.M113.510362. Epub 2013 Oct 22. J Biol Chem. 2013. PMID: 24151072 Free PMC article.
-
Expression and promoter analysis of a highly restricted integrin alpha gene in vascular smooth muscle.Gene. 2013 Jan 15;513(1):82-9. doi: 10.1016/j.gene.2012.10.073. Epub 2012 Nov 8. Gene. 2013. PMID: 23142384 Free PMC article.
References
-
- Birukov KG, Schavocky JP, Shirinsky VP, Chibalina MV, Van Eldik LJ, Watterson DM. Organization of the genetic locus for chicken myosin light chain kinase is complex: multiple proteins are encoded and exhibit differential expression and localization. J Cell Biochem. 1998;70:402–413. - PubMed
-
- Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol. 1976;47:137–194. - PubMed
-
- Cunha GR, Young P, Brody JR. Role of uterine epithelium in the development of myometrial smooth muscle cells. Biol Reprod. 1989;40:861–871. - PubMed
-
- Eddinger TJ, Wolf JA. Expression of four myosin heavy chain isoforms with development in mouse uterus. Cell Motil Cytoskeleton. 1993;25:358–368. - PubMed
-
- Fisher SA, Ikebe M. Developmental and tissue distribution of expression of nonmuscle and smooth muscle isoforms of myosin light chain kinase. Biochem Biophys Res Commun. 1995;217:696–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

