N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility
- PMID: 11121435
- PMCID: PMC2190584
- DOI: 10.1083/jcb.151.6.1193
N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility
Abstract
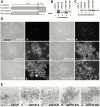
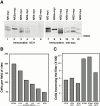
E- and N-cadherin are members of the classical cadherin family of proteins. E-cadherin plays an important role in maintaining the normal phenotype of epithelial cells. Previous studies from our laboratory and other laboratories have shown that inappropriate expression of N-cadherin by tumor cells derived from epithelial tissue results in conversion of the cell to a more fibroblast-like cell, with increased motility and invasion. Our present study was designed to determine which domains of N-cadherin make it different from E-cadherin, with respect to altering cellular behavior, such as which domains are responsible for the epithelial to mesenchymal transition and increased cell motility and invasion. To address this question, we constructed chimeric cadherins comprised of selected domains of E- and N-cadherin. The chimeras were transfected into epithelial cells to determine their effect on cell morphology and cellular behavior. We found that a 69-amino acid portion of EC-4 of N-cadherin was necessary and sufficient to promote both an epithelial to mesenchymal transition in squamous epithelial cells and increased cell motility. Here, we show that different cadherin family members promote different cellular behaviors. In addition, we identify a novel activity that can be ascribed to the extracellular domain of N-cadherin.
Figures
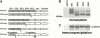
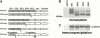
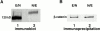


References
-
- Behrens J. Cadherins and cateninsrole in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. - PubMed
-
- Blaschuk O.W., Sullivan R., David S., Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev. Biol. 1990;139:227–229. - PubMed
-
- Byers S., Amaya E., Munro S., Blaschuk O. Fibroblast growth factor receptors contain a conserved HAV region common to cadherins and influenza strain A hemagglutininsa role in protein–protein interactions? Dev. Biol. 1992;152:411–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

