Quantitative analysis of globin gene induction in single human erythroleukemic cells
- PMID: 11121491
- PMCID: PMC115244
- DOI: 10.1093/nar/28.24.4998
Quantitative analysis of globin gene induction in single human erythroleukemic cells
Abstract
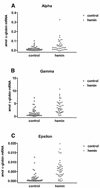
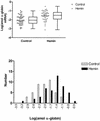
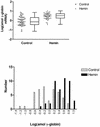
The mechanisms involved in the normal developmental regulation of globin gene expression, and the response to pharmacological agents that elevate fetal hemoglobin, may be expected to involve either changes in each cell or a selection process affecting subsets of differentiating erythroid cells. To study these mechanisms we have developed assays to measure mRNA levels in single erythroid cells. The assay involved the use of globin-specific probes, with no detectable cross-reactivity, in real-time, fluorescence-based quantitative PCR (Q-PCR). We had previously used this Q-PCR method to measure globin mRNA levels in cultures of primary erythroid cells demonstrating that drugs like hydroxyurea, 5-azacytidine and butyric acid each yielded increases in gamma/( gamma + ss) mRNA ratios, with differential effects on ss-globin levels. We have now extended this approach to measure globin mRNA levels in single K562 cells, a human erythroleukemic cell line, with and without 30 microM hemin treatment. Hemin exposure increases total hemoglobin levels by approximately 9-fold and total alpha-, epsilon- and gamma-globin mRNA levels by 1.5-2.3-fold. Single cell analyses showed initial wide distributions of each of the three individual globin mRNA levels with most cells having detectable but very low levels of each globin transcript. Hemin induction shifted the distributions to higher levels, with a tendency to residual left skewing as some cells remained with very low expression levels despite the effect of hemin in increasing expression in most of these low expressing cells. Thus transcriptional heterogeneity remains a crucial variable, even in this extensively used model of human erythroid biology, and clearly influences strongly the response to inducing agents. These methods may enable us to define better possible molecular and/or cellular models of globin gene modulation.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

