T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB)
- PMID: 11122240
- PMCID: PMC1905783
- DOI: 10.1046/j.1365-2249.2000.01385.x
T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB)
Abstract
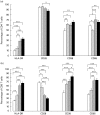
Immune parameters were compared in four groups of Ugandan subjects: HIV-and HIV+ adult patients with active pulmonary TB (HIV- PTB n = 38; HIV+ PTB n = 28), patients with HIV infection only (n = 26) and PPD+ healthy controls (n = 25). Compared with healthy controls, CD4 and CD8 T cells from patients with HIV and/or PTB expressed more activation markers (HLA-DR, CD38); their CD8 T cells expressed more CD95 (pre-apoptosis) and less CD28 (co-stimulatory receptor). Peripheral blood mononuclear cells (PBMC) of patients with either HIV or PTB were impaired in interferon-gamma (IFN-gamma) production upon antigenic stimulation. PTB (with or without HIV) was characterized by monocytosis, granulocytosis, increased transforming growth factor-beta 1 production and PPD-induced apoptosis. In vivo CD4 T cell depletion, in vitro increased spontaneous CD4 T cell apoptosis and defects in IFN-gamma responses upon mitogenic stimulation were restricted to HIV+ subjects (with or without PTB). Overlapping and distinctive immune alterations, associated with PTB and HIV, might explain mutual unfavourable influences of both diseases.
Figures


Comment in
-
Partners in crime: co-infections in the developing world.Clin Exp Immunol. 2000 Dec;122(3):296-9. doi: 10.1046/j.1365-2249.2000.01407.x. Clin Exp Immunol. 2000. PMID: 11122231 Free PMC article. Review. No abstract available.
Similar articles
-
Specific cytokine patterns of pulmonary tuberculosis in Central Africa.Clin Immunol. 2011 Jan;138(1):50-9. doi: 10.1016/j.clim.2010.09.005. Epub 2010 Oct 15. Clin Immunol. 2011. PMID: 20951096
-
Interferon-gamma and tumour necrosis factor-alpha production by CD4+ T and CD8+ T lymphocytes in AIDS patients with tuberculosis.Clin Exp Immunol. 2005 Jun;140(3):491-7. doi: 10.1111/j.1365-2249.2005.02796.x. Clin Exp Immunol. 2005. PMID: 15932510 Free PMC article.
-
Macrophage-activating cytokines in human immununodeficiency virus type 1-infected and -uninfected patients with pulmonary tuberculosis.J Infect Dis. 2001 Jun 15;183(12):1805-9. doi: 10.1086/320725. Epub 2001 May 17. J Infect Dis. 2001. PMID: 11372035
-
Examining a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy.Tuber Lung Dis. 1997;78(3-4):145-58. doi: 10.1016/s0962-8479(97)90021-6. Tuber Lung Dis. 1997. PMID: 9713647 Review.
-
Partners in crime: co-infections in the developing world.Clin Exp Immunol. 2000 Dec;122(3):296-9. doi: 10.1046/j.1365-2249.2000.01407.x. Clin Exp Immunol. 2000. PMID: 11122231 Free PMC article. Review. No abstract available.
Cited by
-
Male Sex Is Associated With Worse Microbiological and Clinical Outcomes Following Tuberculosis Treatment: A Retrospective Cohort Study, a Systematic Review of the Literature, and Meta-analysis.Clin Infect Dis. 2021 Nov 2;73(9):1580-1588. doi: 10.1093/cid/ciab527. Clin Infect Dis. 2021. PMID: 34100919 Free PMC article.
-
Mechanistic insights on immunosenescence and chronic immune activation in HIV-tuberculosis co-infection.World J Virol. 2015 Feb 12;4(1):17-24. doi: 10.5501/wjv.v4.i1.17. World J Virol. 2015. PMID: 25674514 Free PMC article. Review.
-
Heightened Microbial Translocation Is a Prognostic Biomarker of Recurrent Tuberculosis.Clin Infect Dis. 2022 Nov 14;75(10):1820-1826. doi: 10.1093/cid/ciac236. Clin Infect Dis. 2022. PMID: 35352112 Free PMC article.
-
Enhancement of human antigen-specific memory T-cell responses by interleukin-7 may improve accuracy in diagnosing tuberculosis.Clin Vaccine Immunol. 2008 Oct;15(10):1616-22. doi: 10.1128/CVI.00185-08. Epub 2008 Aug 27. Clin Vaccine Immunol. 2008. PMID: 18753334 Free PMC article.
-
Systemic immune activation and microbial translocation in dual HIV/tuberculosis-infected subjects.J Infect Dis. 2013 Jun 15;207(12):1841-9. doi: 10.1093/infdis/jit092. Epub 2013 Mar 11. J Infect Dis. 2013. PMID: 23479321 Free PMC article. Clinical Trial.
References
-
- Kaplan G, Freedman VH. The role of cytokines in the immune response to tuberculosis. Res Immunol. 1996;147:565–72. - PubMed
-
- Rich EA. Pulmonary immune response to Mycobacterium tuberculosis and human immunodeficiency virus. Infect Agents Dis. 1996;5:108–18. - PubMed
-
- Vanham G, Toossi Z, Hirsch CS, Wallis RS, Schwander SK, Rich EA, Ellner JJ. Examining a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy. Tuber Lung Dis. 1997;78:145–58. - PubMed
-
- Toossi Z. Cytokine circuits in tuberculosis. Infect Agents Dis. 1996;5:98–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

