Expression of cyclooxygenase-1 (COX-1) in labial salivary glands of Sjögren's syndrome
- PMID: 11122255
- PMCID: PMC1905792
- DOI: 10.1046/j.1365-2249.2000.01302.x
Expression of cyclooxygenase-1 (COX-1) in labial salivary glands of Sjögren's syndrome
Abstract
COX plays an important role in inflammatory diseases such as rheumatoid arthritis. To determine the role of COX in Sjögren's syndrome (SS), we examined COX expression in the salivary glands of SS patients. We examined 15 patients with SS and two normal subjects. Labial salivary gland tissue samples were analysed immunohistochemically using anti-COX-1 and COX-2 antibodies. All biopsy samples from 15 patients with SS were stained for COX-1. In contrast, COX-1 immunostaining was not detected in normal salivary gland tissues. Co-expression of COX-1 and CD68 was confirmed by mirror section technique and double antibody immunostaining. This finding indicated that COX-1-expressing cells in SS salivary glands were infiltrating macrophages. In contrast to COX-1 staining, only a little COX-2 immunostaining was observed in salivary gland tissues from SS patients. These data suggest that COX-1 expression on infiltrating macrophages may contribute to the inflammatory process of salivary glands in SS.
Figures
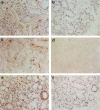


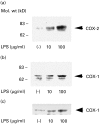
Similar articles
-
Characteristics of Labial Gland Mesenchymal Stem Cells of Healthy Individuals and Patients with Sjögren's Syndrome: A Preliminary Study.Stem Cells Dev. 2017 Aug 15;26(16):1171-1185. doi: 10.1089/scd.2017.0045. Epub 2017 Jun 26. Stem Cells Dev. 2017. PMID: 28537471
-
Expression of mitogen activated protein kinases in labial salivary glands of patients with Sjögren's syndrome.Ann Rheum Dis. 1999 Jun;58(6):382-5. doi: 10.1136/ard.58.6.382. Ann Rheum Dis. 1999. PMID: 10340964 Free PMC article.
-
Activated caspase 3 and cleaved poly(ADP-ribose)polymerase in salivary epithelium suggest a pathogenetic mechanism for Sjögren's syndrome.Rheumatology (Oxford). 2002 Mar;41(3):338-42. doi: 10.1093/rheumatology/41.3.338. Rheumatology (Oxford). 2002. PMID: 11934973
-
Salivary gland biopsy for Sjögren's syndrome.Oral Maxillofac Surg Clin North Am. 2014 Feb;26(1):23-33. doi: 10.1016/j.coms.2013.09.005. Oral Maxillofac Surg Clin North Am. 2014. PMID: 24287191 Review.
-
Salivary histopathology in diagnosis of Sjögren's syndrome.Scand J Rheumatol Suppl. 1986;61:36-43. Scand J Rheumatol Suppl. 1986. PMID: 3296156 Review.
Cited by
-
Horizons in Sjögren's syndrome genetics.Clin Rev Allergy Immunol. 2007 Jun;32(3):201-9. doi: 10.1007/s12016-007-8002-9. Clin Rev Allergy Immunol. 2007. PMID: 17963047 Free PMC article. Review.
-
The genetics of primary Sjögren's syndrome.Curr Rheumatol Rep. 2003 Aug;5(4):324-32. doi: 10.1007/s11926-003-0012-x. Curr Rheumatol Rep. 2003. PMID: 14531961 Review.
-
Case report: Clinical and immunohistochemical manifestations of suspected Sjogren's disease in a dog.Front Vet Sci. 2024 Nov 27;11:1479363. doi: 10.3389/fvets.2024.1479363. eCollection 2024. Front Vet Sci. 2024. PMID: 39711802 Free PMC article.
References
-
- O'Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Letters. 1993;330:156–60. - PubMed
-
- Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxidase synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993;268:9049–54. - PubMed
-
- Dewitt DL, Maeda EA. Serum and glucocorticoid regulation of gene transcription and expression of prostaglandin H synthase-1 and prostaglandin H synthase-2 isozymes. Arch Biochem Biophys. 1993;306:94–102. - PubMed
-
- Ueda N, Yamashita R, Yamamoto S, Ishimura K. Induction of cyclooxygenase-1 in a human megakaryoblastic cell line (CMK) differentiated by phorbol ester. Biochim Biophys Acta. 1997;1344:103–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

