A critical role for alveolar macrophages in elicitation of pulmonary immune fibrosis
- PMID: 11122454
- PMCID: PMC2327107
- DOI: 10.1046/j.1365-2567.2000.00143.x
A critical role for alveolar macrophages in elicitation of pulmonary immune fibrosis
Abstract
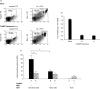
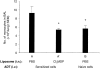
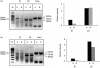
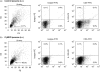
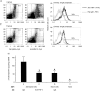
Hapten immune pulmonary interstitial fibrosis (HIPIF) is induced by a recall cell-mediated immune response against the hapten 2,4, 6-trinitrobenzene sulphonic acid (TNBS) in the lung. Studies here dissect the role of the cellular components of the bronchoalveolar lavage (BAL) cells (alveolar macrophages [AMs] versus monocytes and immature dendritic cells) in the fibrogenic inflammatory response. BAL cells from HIPIF mice were generally more activated and produced a greater amount of tumour necrosis factor-alpha (TNF-alpha) than controls. Liposome-encapsulated dichloromethylene diphosphonate (Cl(2)MDP) that was inoculated intranasally (i.n.) into mice selectively depleted AMs. Following AM depletion, the number of TNF-alpha-containing cells was reduced, and both the number of immune inflammatory cells recruited into the alveolar space and the subsequent collagen deposition (hydroxyproline) were decreased in the sensitized and intratracheally (i.t.) challenged mice. In conclusion, AMs are required, in part, for the development of pulmonary fibrosis in HIPIF because AM-derived factors such as TNF-alpha are needed for initiation of chemokine and cytokine pathways and accumulation of immune inflammatory cells.
Figures

References
-
- Weltzien HU, Moulon C, Marin C, Padovan S, Harmann U, Kohler J. T cell immune response to haptens. Structural models for allergic and autoimmune reactions. Toxicology. 1996;107:141–51. 10.1016/0300-483x(95)03253-c. - DOI - PubMed
-
- Kalish RS, Wood JA. Induction of hapten-specific tolerance of human CD8+ urushiol (poison ivy)-reactive T lymphocytes. J Invest Dermatol. 1997;108:253–7. - PubMed
-
- Gelber C, Gemmell L, McAteer D, et al. Down-regulation of poison ivy/oak-induced contact sensitivity by treatment with a class II MHC binding peptide–hapten conjugate. J Immunol. 1997;158:2425–35. - PubMed
-
- Padovan E, von Greyerz S, Pichler WJ, Weltzien HU. Antigen-dependent and -independent IFN-gamma modulation by penicillins. J Immunol. 1999;162:1171–7. - PubMed
-
- Coleman JW. Protein haptenation by drugs. Clin Exp Allergy. 1998;28:79–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

