Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization
- PMID: 11124805
- PMCID: PMC317135
- DOI: 10.1101/gad.182800
Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization
Abstract
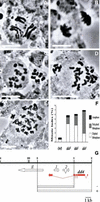
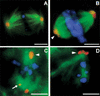
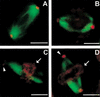
We have cloned the Drosophila gene discs degenerate-4 (dd4) and find that it encodes a component of the gamma-tubulin ring complex (gammaTuRC) homologous to Spc98 of budding yeast. This provides the first opportunity to study decreased function of a member of the gamma-tubulin ring complex, other than gamma-tubulin itself, in a metazoan cell. gamma-tubulin is no longer at the centrosomes but is dispersed throughout dd4 cells and yet bipolar metaphase spindles do form, although these have a dramatically decreased density of microtubules. Centrosomin (CNN) remains in broad discrete bodies but only at the focused poles of such spindles, whereas Asp (abnormal spindle protein) is always present at the presumptive minus ends of microtubules, whether or not they are focused. This is consistent with the proposed role of Asp in coordinating the nucleation of mitotic microtubule organizing centers. The centrosome associated protein CP190 is partially lost from the spindle poles in dd4 cells supporting a weak interaction with gamma-tubulin, and the displaced protein accumulates in the vicinity of chromosomes. Electron microscopy indicates not only that the poles of dd4 cells have irregular amounts of pericentriolar material, but also that they can have abnormal centrioles. In six dd4 cells subjected to serial sectioning centrioles were missing from one of the two poles. This suggests that in addition to its role in nucleating cytoplasmic and spindle microtubules, the gammaTuRC is also essential to the structure of centrioles and the separation of centrosomes.
Figures




References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York: John Wiley & Sons; 1999.
-
- Avides MCO, Glover DM. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 1999;283:1733–1735. - PubMed
-
- Axton JM. &rldquo;Genetic and molecular analysis of mitotic chromosome condensation in Drosophila”. Ph.D. thesis. London, UK: Imperial College of Science and Technology; 1990.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
