Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses
- PMID: 11124810
- PMCID: PMC317149
- DOI: 10.1101/gad.853700
Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses
Abstract
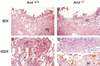
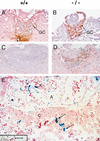
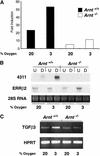
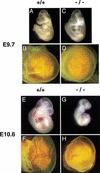
Placental development is profoundly influenced by oxygen (O(2)) tension. Human cytotrophoblasts proliferate in vitro under low O(2) conditions but differentiate at higher O(2) levels, mimicking the developmental transition they undergo as they invade the placental bed to establish the maternal-fetal circulation in vivo. Hypoxia-inducible factor-1 (HIF-1), consisting of HIF-1alpha and ARNT subunits, activates many genes involved in the cellular and organismal response to O(2) deprivation. Analysis of Arnt(-/-) placentas reveals an aberrant cellular architecture due to altered cell fate determination of Arnt(-/-) trophoblasts. Specifically, Arnt(-/-) placentas show greatly reduced labyrinthine and spongiotrophoblast layers, and increased numbers of giant cells. We further show that hypoxia promotes the in vitro differentiation of trophoblast stem cells into spongiotrophoblasts as opposed to giant cells. Our results clearly establish that O(2) levels regulate cell fate determination in vivo and that HIF is essential for mammalian placentation. The unique placental phenotype of Arnt(-/-) animals also provides an important tool for studying the disease of preeclampsia. Interestingly, aggregation of Arnt(-/-) embryonic stem (ES) cells with tetraploid wild-type embryos rescues their placental defects; however, these embryos still die from yolk sac vascular and cardiac defects.
Figures



References
-
- Akazawa S, Unterman T, Metzger BE. Glucose metabolism in separated embryos and investing membranes during organogenesis in the rat. Metabolism. 1994;43:830–835. - PubMed
-
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature. 1998;392:405–408. - PubMed
-
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
