Water permeability of cochlear outer hair cells: characterization and relationship to electromotility
- PMID: 11124975
- PMCID: PMC6773017
- DOI: 10.1523/JNEUROSCI.20-24-08996.2000
Water permeability of cochlear outer hair cells: characterization and relationship to electromotility
Abstract
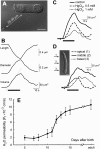
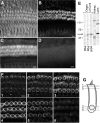
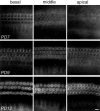
The distinguishing feature of the mammalian outer hair cells (OHCs) is to elongate and shorten at acoustic frequencies, when their intracellular potential is changed. This "electromotility" or "electromechanics" depends critically on positive intracellular pressure (turgor), maintained by the inflow of water through yet uncharacterized water pathways. We measured the water volume flow, J(v), across the plasma membrane of isolated guinea pig and rat OHCs after osmotic challenges and estimated the osmotic water permeability coefficient, P(f), to be approximately 10(-2) cm/sec. This value is within the range reported for osmotic flow mediated by the water channel proteins, aquaporins. J(v) was inhibited by HgCl(2), which is known to block aquaporin-mediated water transport. P(f) values that were estimated for OHCs from neonatal rats were of the order of approximately 2 x 10(-3) cm/sec, equivalent to that of membranes lacking water channel proteins. In an immunofluorescence assay we showed that an anti-peptide antibody specific for aquaporins labels the lateral plasma membrane of the OHC in the region in which electromotility is generated. Using patch-clamp recording, we found that water influx into the OHC is regulated by intracellular voltage. We also found that the most pronounced increases of the electromotility-associated charge movement and of the expression of OHC water channels occur between postnatal days 8 and 12, preceding the onset of hearing function in the rat. Our data indicate that electromotility and water transport in OHCs may influence each other structurally and functionally.
Figures


References
-
- Beitz E, Kumagami H, Krippeit-Drews P, Ruppersberg JP, Schultz JE. Expression pattern of aquaporin water channels in the inner ear of the rat. The molecular basis for a water regulation system in the endolymphatic sac. Hear Res. 1999;132:76–84. - PubMed
-
- Belyantseva IA, Frolenkov GI, Streett D, Wade J, Kachar B. Is the voltage-driven motor protein for outer hair cell electromotility a member of the aquaporin protein family? Abstracts of 22nd Meeting of the Association for Research in Otolaryngology Popelka GR. 1999, abstract 731. St. Petersburg Beach, FL: ARO.
-
- Brown D, Katsura T, Kawashima M, Verkman AS, Sabolic I. Cellular distribution of the aquaporins: a family of water channel proteins. Histochem Cell Biol. 1995;104:1–9. - PubMed
-
- Butkus A, Alcorn D, Earnest L, Moritz K, Giles M, Wintour EM. Expression of aquaporin-1 (AQP1) in the adult and developing sheep kidney. Biol Cell. 1997;89:313–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
