NBQX attenuates excitotoxic injury in developing white matter
- PMID: 11125001
- PMCID: PMC6773002
- DOI: 10.1523/JNEUROSCI.20-24-09235.2000
NBQX attenuates excitotoxic injury in developing white matter
Erratum in
- J Neurosci 2001 Mar 1;21(5):1a
Abstract
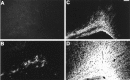
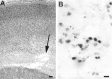
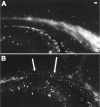
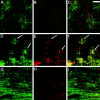
The excitatory neurotransmitter glutamate is released from axons and glia under hypoxic/ischemic conditions. In vitro, oligodendrocytes (OLs) express non-NMDA glutamate receptors (GluRs) and are susceptible to GluR-mediated excitotoxicity. We evaluated the role of GluR-mediated OL excitotoxicity in hypoxic/ischemic white matter injury in the developing brain. Hypoxic/ischemic white matter injury is thought to mediate periventricular leukomalacia, an age-dependent white matter lesion seen in preterm infants and a common antecedent to cerebral palsy. Hypoxia/ischemia in rat pups at postnatal day 7 (P7) produced selective white matter lesions and OL death. Furthermore, OLs in pericallosal white matter express non-NMDA GluRs at P7. Unilateral carotid ligation in combination with hypoxia (6% O(2) for 1 hr) resulted in selective, subcortical white matter injury with a marked ipsilateral decrease in immature and myelin basic protein-expressing OLs that was also significantly attenuated by 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX). Intracerebral AMPA demonstrated greater susceptibility to OL injury at P7 than in younger or older pups, and this was attenuated by systemic pretreatment with the AMPA antagonist NBQX. These results indicate a parallel, maturation-dependent susceptibility of immature OLs to AMPA and hypoxia/ischemia. The protective efficacy of NBQX suggests a role for glutamate receptor-mediated excitotoxic OL injury in immature white matter in vivo.
Figures



References
-
- Andine P, Thordstein M, Kjellmer I, Nordborg C, Thiringer K, Wennberg E, Hagberg H. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–260. - PubMed
-
- Asou H, Hamada K, Miyazaki T, Sakota T, Hayashi K, Takeda Y, Marret S, Delpech B, Itah K, Uyemura K. CNS myelination in vitro: time course and pattern of rat oligodendrocyte development. J Neurosci Res. 1995;40:519–534. - PubMed
-
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;4:1369–1374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
