Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue
- PMID: 11125004
- PMCID: PMC6773001
- DOI: 10.1523/JNEUROSCI.20-24-09264.2000
Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue
Abstract
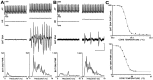
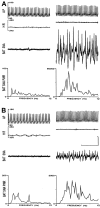
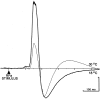
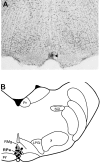
Sympathetic outflow to brown adipose tissue (BAT) contributes to both thermoregulation and energy expenditure in rats through regulation of BAT thermogenesis. Acute cold exposure in mature animals augments BAT thermogenesis; however, the enhanced BAT thermogenic response returns to normal shortly after cessation of the cold exposure. In this study, we sought to determine whether cold exposure in early neonatal life could induce enhanced responses in the sympathetic outflow to BAT and whether this altered sympathetic regulation would be sustained after the cold stimulus was removed. BAT sympathetic nerve activity (SNA) was recorded in urethane-chloralose-anesthetized, artificially ventilated rats that were raised from birth in either 18 or 30 degrees C environments and then, at 8 weeks of age, were maintained in 23 degrees C for at least 4 weeks. An acute hypothermic stimulus, disinhibition of a brainstem thermogenic network in the raphe pallidus, or electrical stimulation in this raphe site produced increases in BAT SNA that were twice as great in rats reared at 18 degrees C as in those reared at 30 degrees C. The norepinephrine content of the interscapular BAT (IBAT) and the number of sympathetic ganglion cells projecting to interscapular BAT were 70% greater in the 18 degrees C-reared rats. We conclude that neonatal exposure to a cold environment induces a permanent developmental alteration in the capacity for sympathetic stimulation of BAT thermogenesis that may be mediated, in part, by a greater number of sympathetic ganglion cells innervating BAT in cold-reared animals.
Figures
Similar articles
-
GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue.Am J Physiol. 1999 Feb;276(2):R290-7. doi: 10.1152/ajpregu.1999.276.2.R290. Am J Physiol. 1999. PMID: 9950904
-
Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue.Am J Physiol Regul Integr Comp Physiol. 2009 Mar;296(3):R831-43. doi: 10.1152/ajpregu.91007.2008. Epub 2009 Jan 7. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19129373 Free PMC article.
-
Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue.Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R127-36. doi: 10.1152/ajpregu.00427.2006. Epub 2006 Aug 24. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 16931649 Free PMC article.
-
Sympathetic and sensory innervation of brown adipose tissue.Int J Obes (Lond). 2010 Oct;34 Suppl 1(0 1):S36-42. doi: 10.1038/ijo.2010.182. Int J Obes (Lond). 2010. PMID: 20935665 Free PMC article. Review.
-
Does thermoregulatory feeding occur in newborn infants? A novel view of the role of brown adipose tissue thermogenesis in control of food intake.Obes Res. 1995 Jul;3(4):361-9. doi: 10.1002/j.1550-8528.1995.tb00162.x. Obes Res. 1995. PMID: 8521153 Review.
Cited by
-
Brown fat thermogenesis and cold adaptation in humans.J Physiol Anthropol. 2025 Apr 21;44(1):11. doi: 10.1186/s40101-025-00391-w. J Physiol Anthropol. 2025. PMID: 40259336 Free PMC article. Review.
-
Obesity: a neuroimmunometabolic perspective.Nat Rev Endocrinol. 2020 Jan;16(1):30-43. doi: 10.1038/s41574-019-0283-6. Epub 2019 Nov 27. Nat Rev Endocrinol. 2020. PMID: 31776456 Review.
-
A Tale of Three Systems: Toward a Neuroimmunoendocrine Model of Obesity.Annu Rev Cell Dev Biol. 2021 Oct 6;37:549-573. doi: 10.1146/annurev-cellbio-120319-114106. Annu Rev Cell Dev Biol. 2021. PMID: 34613819 Free PMC article. Review.
-
Ancestral and developmental cold alter brown adipose tissue function and adult thermal acclimation in Peromyscus.J Comp Physiol B. 2021 May;191(3):589-601. doi: 10.1007/s00360-021-01355-z. Epub 2021 Mar 1. J Comp Physiol B. 2021. PMID: 33644836
-
Neural driven angiogenesis by overexpression of nerve growth factor.Histochem Cell Biol. 2006 Jun;125(6):637-49. doi: 10.1007/s00418-005-0111-z. Epub 2005 Nov 29. Histochem Cell Biol. 2006. PMID: 16315017
References
-
- Aguayo AJ, Peyronnard JM, Terry LC, Romine JS, Bray GM. Neonatal neuronal loss in rat superior cervical ganglia: retrograde effects on developing preganglionic axons and Schwann cells. J Neurocytol. 1976;5:137–155. - PubMed
-
- Bertin R, Mouroux I, De Marco F, Portet R. Norepinephrine turnover in brown adipose tissue of young rats: effects of rearing temperature. Am J Physiol. 1990;259:R90–R96. - PubMed
-
- Boulant JA. Hypothalamic control of thermoregulation. In: Morgane PJ, Panksepp J, editors. The handbook of the hypothalamus. Dekker; New York: 1980. pp. 1–82.
-
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. - PubMed
-
- Brunjes PC, Frazier LL. Maturation and plasticity in the olfactory system of vertebrates. Brain Res Rev. 1986;11:1–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
