Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant
- PMID: 11133472
- PMCID: PMC92592
- DOI: 10.1128/AEM.67.1.403-410.2001
Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant
Erratum in
- Appl Environ Microbiol 2001 Nov;67(11):5349
Abstract
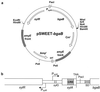
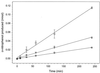
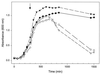
We have developed a xylose-dependent expression system for tight and modulated expression of cloned genes in Bacillus subtilis. The expression system is contained on plasmid pSWEET for integration at the amyE locus of B. subtilis and incorporates components of the well-characterized, divergently transcribed xylose utilization operon. The system contains the xylose repressor encoded by xylR, the promoter and 5' portion of xylA containing an optimized catabolite-responsive element, and intergenic xyl operator sequences. We have rigorously compared this expression system to the isopropyl-beta-D-thiogalactopyranoside-induced spac system using a thermostable beta-galactosidase reporter (BgaB) and found the xyl promoter-operator to have a greater capacity for modulated expression, a higher induction/repression ratio (279-fold for the xyl system versus 24-fold with the spac promoter), and lower levels of expression in the absence of an inducer. We have used this system to probe an essential function in wall teichoic acid biosynthesis in B. subtilis. Expression of the teichoic acid biosynthesis gene tagD, encoding glycerol-3-phosphate cytidylyltransferase, from the xylose-based expression system integrated at amyE exhibited xylose-dependent complementation of the temperature-sensitive mutant tag-12 when grown at the nonpermissive temperature. Plasmid pSWEET thus provides a robust new expression system for conditional complementation in B. subtilis.
Figures



References
-
- Briehl M, Pooley H M, Karamata D. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid biosynthesis. J Gen Microbiol. 1989;135:1325–1334.
-
- Cutting S M, Horn P B V. Genetic analysis. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Toronto, Canada: John Wiley and Sons; 1990. pp. 27–61.
-
- Cutting S M, Youngman P. Gene transfer in gram-positive bacteria. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 348–364.
-
- Dahl M K, Degenkolb J, Hillen W. Transcription of the xyl operon is controlled in Bacillus subtilis by tandem overlapping operators spaced by four base-pairs. J Mol Biol. 1994;243:413–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

