Urkinase: structure of acetate kinase, a member of the ASKHA superfamily of phosphotransferases
- PMID: 11133963
- PMCID: PMC94925
- DOI: 10.1128/JB.183.2.680-686.2001
Urkinase: structure of acetate kinase, a member of the ASKHA superfamily of phosphotransferases
Abstract
Acetate kinase, an enzyme widely distributed in the Bacteria and Archaea domains, catalyzes the phosphorylation of acetate. We have determined the three-dimensional structure of Methanosarcina thermophila acetate kinase bound to ADP through crystallography. As we previously predicted, acetate kinase contains a core fold that is topologically identical to that of the ADP-binding domains of glycerol kinase, hexokinase, the 70-kDa heat shock cognate (Hsc70), and actin. Numerous charged active-site residues are conserved within acetate kinases, but few are conserved within the phosphotransferase superfamily. The identity of the points of insertion of polypeptide segments into the core fold of the superfamily members indicates that the insertions existed in the common ancestor of the phosphotransferases. Another remarkable shared feature is the unusual, epsilon conformation of the residue that directly precedes a conserved glycine residue (Gly-331 in acetate kinase) that binds the alpha-phosphate of ADP. Structural, biochemical, and geochemical considerations indicate that an acetate kinase may be the ancestral enzyme of the ASKHA (acetate and sugar kinases/Hsc70/actin) superfamily of phosphotransferases.
Figures


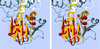

Similar articles
-
Crystal structures of ADP and AMPPNP-bound propionate kinase (TdcD) from Salmonella typhimurium: comparison with members of acetate and sugar kinase/heat shock cognate 70/actin superfamily.J Mol Biol. 2005 Sep 30;352(4):876-92. doi: 10.1016/j.jmb.2005.07.069. J Mol Biol. 2005. PMID: 16139298
-
Site-directed mutational analysis of active site residues in the acetate kinase from Methanosarcina thermophila.J Biol Chem. 2001 Nov 30;276(48):45059-64. doi: 10.1074/jbc.M108355200. Epub 2001 Sep 18. J Biol Chem. 2001. PMID: 11562377
-
Structural and kinetic analyses of arginine residues in the active site of the acetate kinase from Methanosarcina thermophila.J Biol Chem. 2005 Mar 18;280(11):10731-42. doi: 10.1074/jbc.M412118200. Epub 2005 Jan 12. J Biol Chem. 2005. PMID: 15647264
-
The sugar kinase/heat shock protein 70/actin superfamily: implications of conserved structure for mechanism.Annu Rev Biophys Biomol Struct. 1996;25:137-62. doi: 10.1146/annurev.bb.25.060196.001033. Annu Rev Biophys Biomol Struct. 1996. PMID: 8800467 Review.
-
The actin fold.FASEB J. 1995 Feb;9(2):167-74. doi: 10.1096/fasebj.9.2.7781919. FASEB J. 1995. PMID: 7781919 Review.
Cited by
-
Crystal structures of acetate kinases from the eukaryotic pathogens Entamoeba histolytica and Cryptococcus neoformans.J Struct Biol. 2013 Feb;181(2):185-9. doi: 10.1016/j.jsb.2012.11.001. Epub 2012 Nov 16. J Struct Biol. 2013. PMID: 23159802 Free PMC article.
-
Structural and mechanistic investigations on Salmonella typhimurium acetate kinase (AckA): identification of a putative ligand binding pocket at the dimeric interface.BMC Struct Biol. 2012 Oct 2;12:24. doi: 10.1186/1472-6807-12-24. BMC Struct Biol. 2012. PMID: 23031654 Free PMC article.
-
Mechanism of ATP-driven electron transfer catalyzed by the benzene ring-reducing enzyme benzoyl-CoA reductase.Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13619-24. doi: 10.1073/pnas.241375598. Epub 2001 Nov 6. Proc Natl Acad Sci U S A. 2001. PMID: 11698658 Free PMC article.
-
An archaeal orthologue of the universal protein Kae1 is an iron metalloprotein which exhibits atypical DNA-binding properties and apurinic-endonuclease activity in vitro.Nucleic Acids Res. 2007;35(18):6042-51. doi: 10.1093/nar/gkm554. Epub 2007 Aug 30. Nucleic Acids Res. 2007. PMID: 17766251 Free PMC article.
-
Acetate kinase isozymes confer robustness in acetate metabolism.PLoS One. 2014 Mar 17;9(3):e92256. doi: 10.1371/journal.pone.0092256. eCollection 2014. PLoS One. 2014. PMID: 24638105 Free PMC article.
References
-
- Aceti D J, Ferry J G. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. J Biol Chem. 1988;263:15444–15448. - PubMed
-
- Anthony R S, Spector L B. Exchange reactions catalyzed by acetate kinase. J Biol Chem. 1971;246:6129–6135. - PubMed
-
- Anthony R S, Spector L B. A phosphoenzyme intermediary in acetate kinase action. J Biol Chem. 1970;246:6730–6741. - PubMed
-
- Anthony R S, Spector L B. Phosphorylated acetate kinase. Its isolation and reactivity. J Biol Chem. 1972;247:2120–2125. - PubMed
-
- Blättler W A, Knowles J R. Stereochemical course of phosphokinases. The use of adenosine [γ-(S)-16O, 17O, 18O]triphosphate and the mechanistic consequences for the reactions catalyzed by glycerol kinase, hexokinase, pyruvate kinase, and acetate kinase. Biochemistry. 1979;18:3927–3933. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

