Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus
- PMID: 11133967
- PMCID: PMC94929
- DOI: 10.1128/JB.183.2.716-724.2001
Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus
Abstract
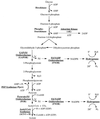
The hyperthermophilic archaeon Pyrococcus furiosus grows optimally at 100 degrees C by the fermentation of peptides and carbohydrates. Growth of the organism was examined in media containing either maltose, peptides (hydrolyzed casein), or both as the carbon source(s), each with and without elemental sulfur (S(0)). Growth rates were highest on media containing peptides and S(0), with or without maltose. Growth did not occur on the peptide medium without S(0). S(0) had no effect on growth rates in the maltose medium in the absence of peptides. Phenylacetate production rates (from phenylalanine fermentation) from cells grown in the peptide medium containing S(0) with or without maltose were the same, suggesting that S(0) is required for peptide utilization. The activities of 14 of 21 enzymes involved in or related to the fermentation pathways of P. furiosus were shown to be regulated under the five different growth conditions studied. The presence of S(0) in the growth media resulted in decreases in specific activities of two cytoplasmic hydrogenases (I and II) and of a membrane-bound hydrogenase, each by an order of magnitude. The primary S(0)-reducing enzyme in this organism and the mechanism of the S(0) dependence of peptide metabolism are not known. This study provides the first evidence for a highly regulated fermentation-based metabolism in P. furiosus and a significant regulatory role for elemental sulfur or its metabolites.
Figures


References
-
- Adams M W W. The biochemical diversity of life near and above 100°C in marine environments. J Appl Microbiol Symp Suppl. 1999;85:108S–117S. - PubMed
-
- Barbier G, Godfroy A, Meunier J-R, Quérellou J, Cambon M-A, Lesongeur F, Grimont P A D, Raguénes G. Pyrococcus glycovorans sp. nov., a hyperthermophilic archaeon isolated from the East Pacific Rise. Int J Syst Bacteriol. 1999;49:1829–1837. - PubMed
-
- Blamey J M, Adams M W W. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim Biophys Acta. 1993;1161:19–27. - PubMed
-
- Blamey J, Chiong M, López C, Smith E. Optimization of the growth conditions of the extremely thermophilic microorganisms Thermococcus celer and Pyrococcus woesei. J Microbiol Methods. 1999;38:169–175. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

