Identification of the outer membrane porin of Thermus thermophilus HB8: the channel-forming complex has an unusually high molecular mass and an extremely large single-channel conductance
- PMID: 11133980
- PMCID: PMC94942
- DOI: 10.1128/JB.183.2.800-803.2001
Identification of the outer membrane porin of Thermus thermophilus HB8: the channel-forming complex has an unusually high molecular mass and an extremely large single-channel conductance
Abstract
The outer membrane of the thermophilic bacterium Thermus thermophilus was isolated using sucrose step gradient centrifugation. Its detergent extracts contained an ion-permeable channel with an extremely high single-channel conductance of 20 nS in 1 M KCl. The channel protein was purified by preparative sodium dodecyl sulfate (SDS)-polyacylamide gel electrophoresis. It has a high molecular mass of 185 kDa, and its channel-forming ability resists boiling in SDS for 10 min.
Figures
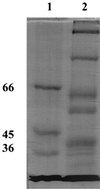
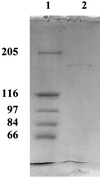

References
-
- Benz R. Solute uptake through bacterial outer membranes. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 397–423.
-
- Benz R, Janko K, Boos W, Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978;511:305–319. - PubMed
-
- Benz R, Schmid A, Maier C, Bremer E. Characterization of the nucleoside-specific binding site inside the Tsx channel of Escherichia coli outer membrane: reconstitution experiments with lipid bilayer membranes. Eur J Biochem. 1988;176:699–705. - PubMed
-
- Benz R, Schmid A, Vos-Scheperkeuter G H. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membrane Biol. 1987;100:21–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

