Temporal and spatial distribution of activated Pak1 in fibroblasts
- PMID: 11134074
- PMCID: PMC2150672
- DOI: 10.1083/jcb.151.7.1449
Temporal and spatial distribution of activated Pak1 in fibroblasts
Abstract
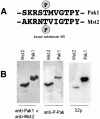
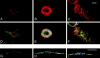
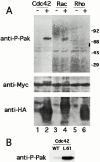
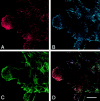
p21-activated kinases (Paks) are effectors of the small GTPases Cdc42 and Rac, and are thought to mediate some of the cytoskeletal and transcriptional activities of these proteins. To localize activated Pak1 in cells, we developed an antibody directed against a phosphopeptide that is contained within the activation loop of Pak1. This antibody specifically recognizes the activated form of Pak1. Immunofluorescence analysis of NIH-3T3 cells coexpressing activated Cdc42 or Rac1 plus wild-type Pak1 shows that activated Pak1 accumulates at sites of focal adhesion, throughout filopodia and within the body and edges of lamellipodia. Platelet-derived growth factor stimulation of NIH-3T3 cells shows a pattern of Pak1 activation similar to that observed with Rac1. During closure of a fibroblast monolayer wound, Pak1 is rapidly activated and localizes to the leading edge of motile cells, then gradually tapers off as the wound closes. The activation of Pak1 by wounding is blocked by inhibitors of phosphatidylinositol 3-kinase, and Src family kinases, but not by an inhibitor of the epidermal growth factor receptor. These findings indicate that activated Pak1, and by extension, probably activated Cdc42 or Rac, accumulates at sites of cortical actin remodeling in motile fibroblasts.
Figures





References
-
- Bagrodia S., Cerione R.A. PAK to the future. Trends Cell Biol. 1999;9:350–355. - PubMed
-
- Bagrodia S., Taylor S., Creasy C.L., Chernoff J., Cerione R.A. Identification of a murine p21Cdc42/Rac activated protein kinase (PAK) J. Biol. Chem. 1995;270:22731–22738. - PubMed
-
- Bokoch G.M., Wang Y., Bohl B.P., Sells M.A., Quilliam L.A., Knaus U.G. Interaction of Nck adapter protein with p21-activated kinase (PAK1) J. Biol. Chem. 1996;271:25746–25749. - PubMed
-
- Chan A.Y., Raft S., Bailly M., Wyckoff J.B., Segall J.E., Condeelis J.S. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J. Cell Sci. 1998;111:199–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

